Free Chemistry NEET Notes for Aldehydes Ketones And Carboxylic Acids
Free Chemistry Notes for Aldehydes Ketones And Carboxylic Acids (NEET)
Power up your NEET Exam prep with Chapter-Wise Chemistry Notes for Aldehydes Ketones And Carboxylic Acids at Onlineneetcoaching.in. Crafted by Brilliant Tutorial experts with 25 years of NEET insights.
- Aldehydes Ketones And Carboxylic Acids -
Important Chemistry Notes for IITJEE/NEET Preparation- Aldehydes Ketones And Carboxylic Acids
Class 12 Chemistry is a broad subject that requires a thorough understanding of the concepts and topics covered. As a result, we have provided Chemistry Notes PDF for IIT JEE/NEET to students and NEET aspirants. Aldehydes Ketones And Carboxylic Acids Class 12 Notes PDF for NEET can be found below. With the help of detailed syllabus, Class 12 students learn what they need to study, how many points are assigned to each unit, and how much time is allotted for each unit. As a result, they can easily plan their study schedule.
Check out the Aldehydes Ketones And Carboxylic Acids Class 12 notes PDF for your IIT JEE/NEET Preparation based on the IIT JEE/NEET Chemistry Syllabus. The Aldehydes Ketones And Carboxylic Acids notes PDF is designed in such a way that it is very useful for IIT JEE/NEET aspirants.
ALDEHYDES AND KETONES
Aldehydes and Ketones are characterised by the presence of Carbonyl group >C = O in their molecules. Aldehydes contain group 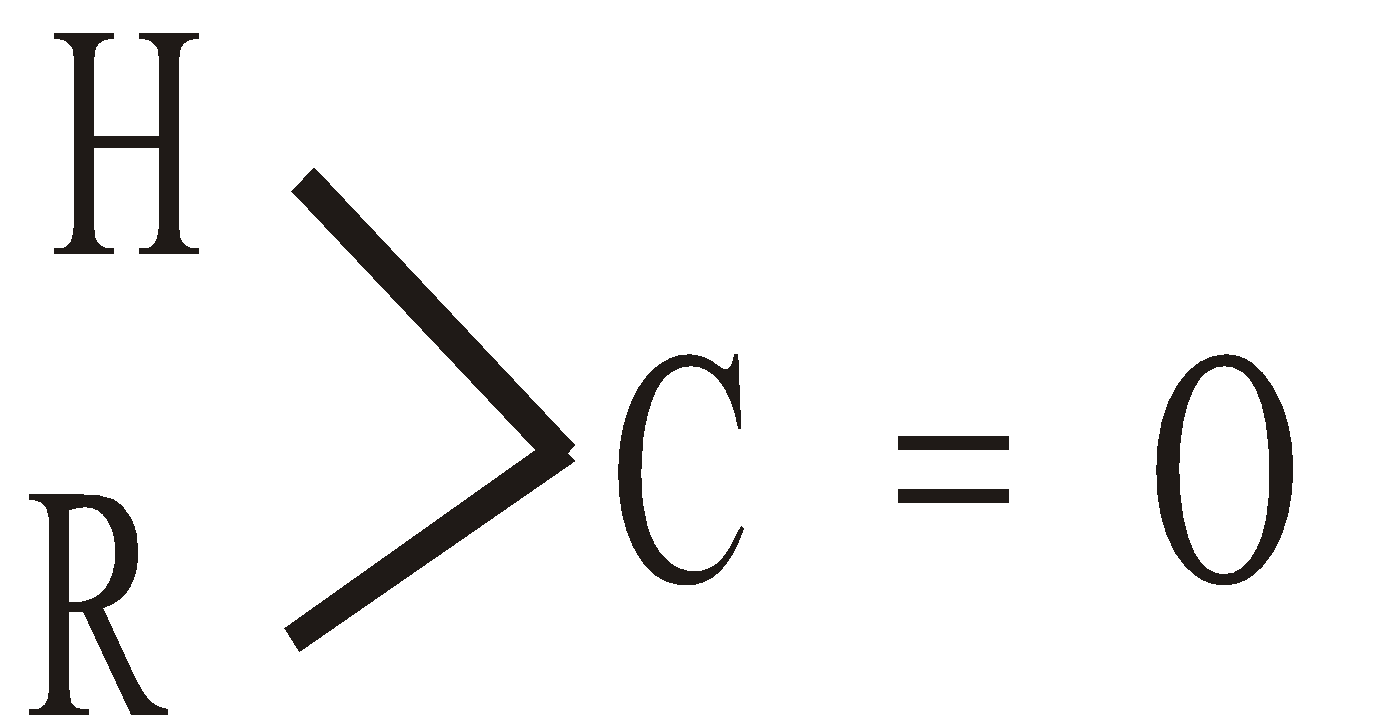 and ketones the
and ketones the 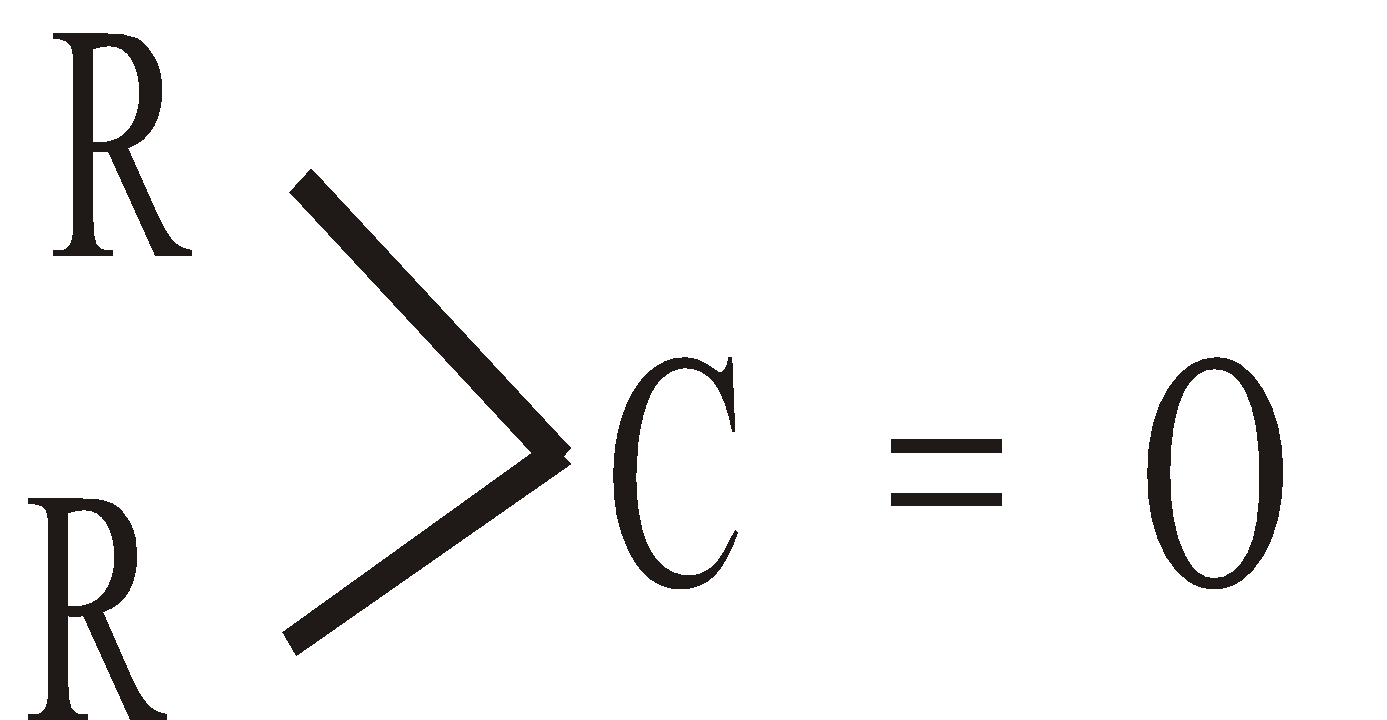 . If the groups attached to carbonyl carbon are the same, the ketone is symmetrical and if they are different the ketone is unsymmetrical.
. If the groups attached to carbonyl carbon are the same, the ketone is symmetrical and if they are different the ketone is unsymmetrical.
NATURE OF CARBONYL GROUP
The carbon and oxygen of the carbonyl group are sp2 hybridised and the carbonyl double bond is made of one s bond and one p bond.
 or
or 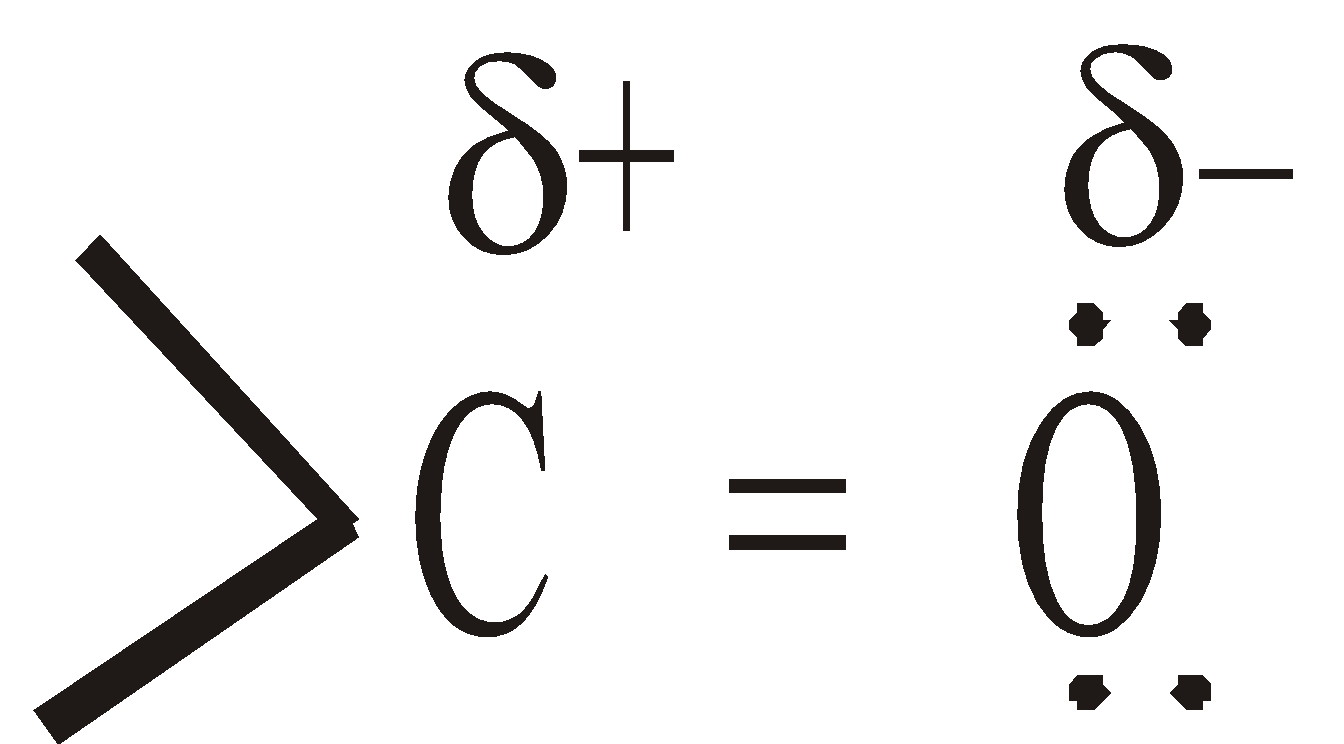 The electronegativity of oxygen is much higher than that of carbon, the p electron cloud is displaced towards the oxygen. Therefore the C–O bond is polar in nature and carbonyl compounds possess dipole moment (2.3 to 2.8 D)
The electronegativity of oxygen is much higher than that of carbon, the p electron cloud is displaced towards the oxygen. Therefore the C–O bond is polar in nature and carbonyl compounds possess dipole moment (2.3 to 2.8 D)
NOMENCLATURE OF ALDEHYDES
There are two systems
-
Common system : The suffix “-ic acid” is replaced by the suffix “-aldehyde”
-
IUPAC system : The suffix “-e” of alkane is replaced by the suffix “-al”.
Compound Common name IUPAC name
HCHO Formaldehyde Methanal
CH3CHO Acetaldehyde Ethanal
NOMENCLATURE OF KETONES
-
Common system : Symmetrical ketones are named as dialkyl ketone and name of unsymmetrical ketone is obtained by naming the alkyl groups alphabetically and adding the third word ketone.
-
IUPAC system : The suffix “-e” of corresponding alkane is replaced by “-one”
Compound Common name IUPAC name
H3C.COCH3 dimethyl ketone (acetone) Propanone
H3C.COC2H5 ethyl methyl ketone butanone
In higher ketones the numbering of C-atoms is must to show the position of carbonyl group. eg. :
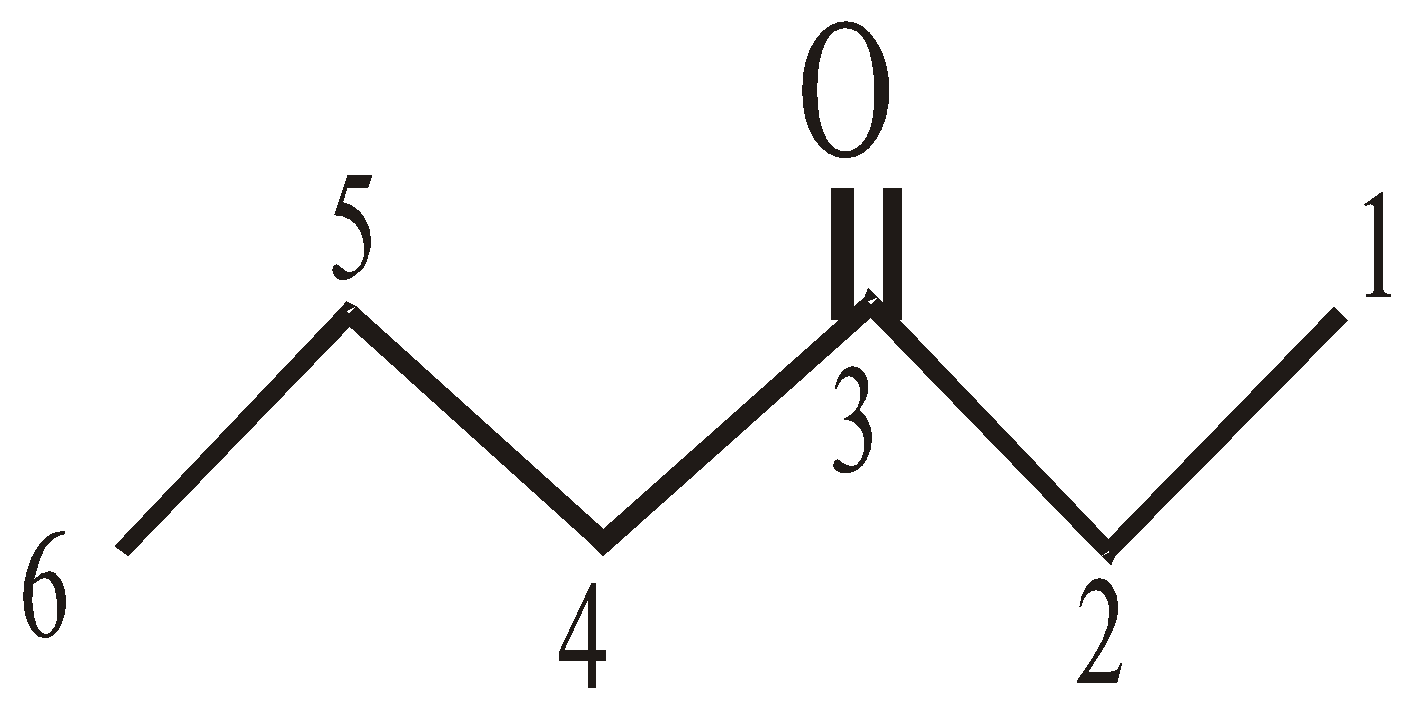 3-hexanone
3-hexanone
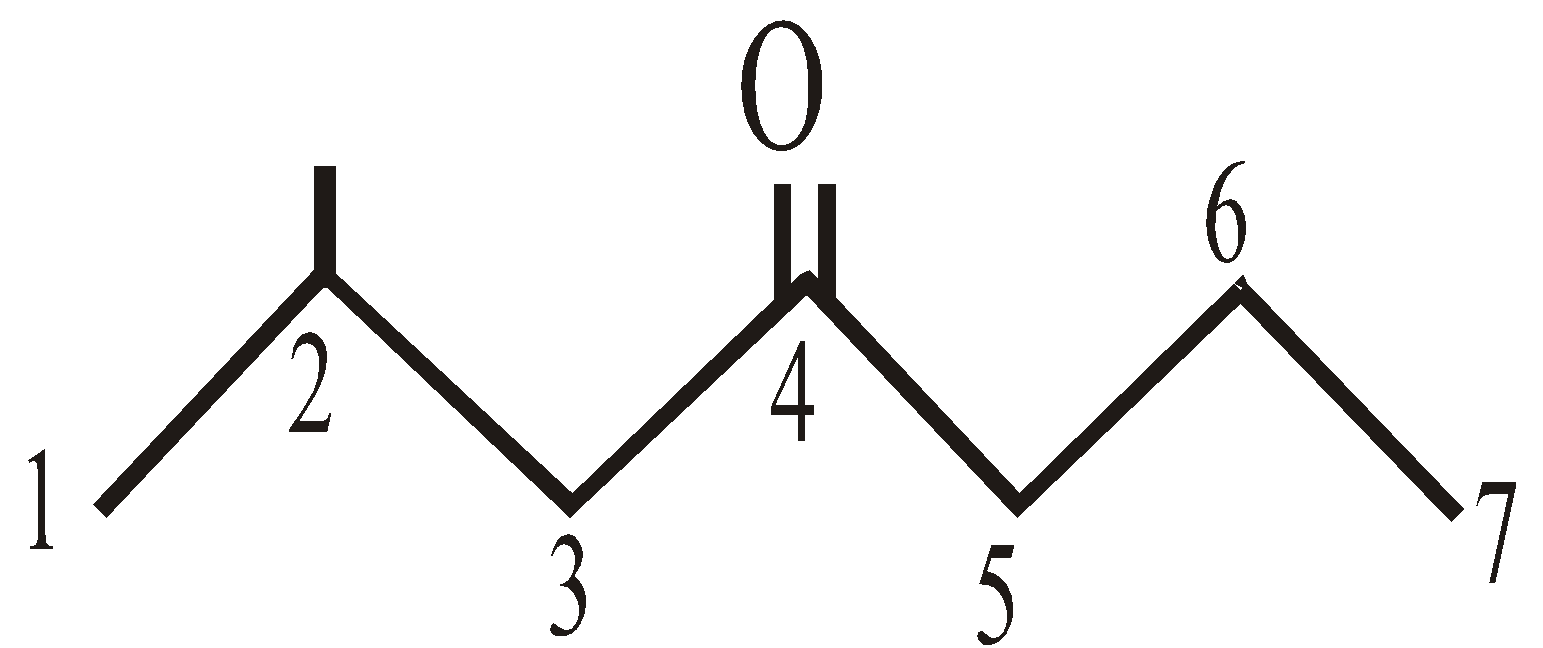 2-methyl-4-heptanone
2-methyl-4-heptanone
ISOMERISM IN ALDEHYDES
Aldehydes exhibit two types of isomerism
Chain isomerism

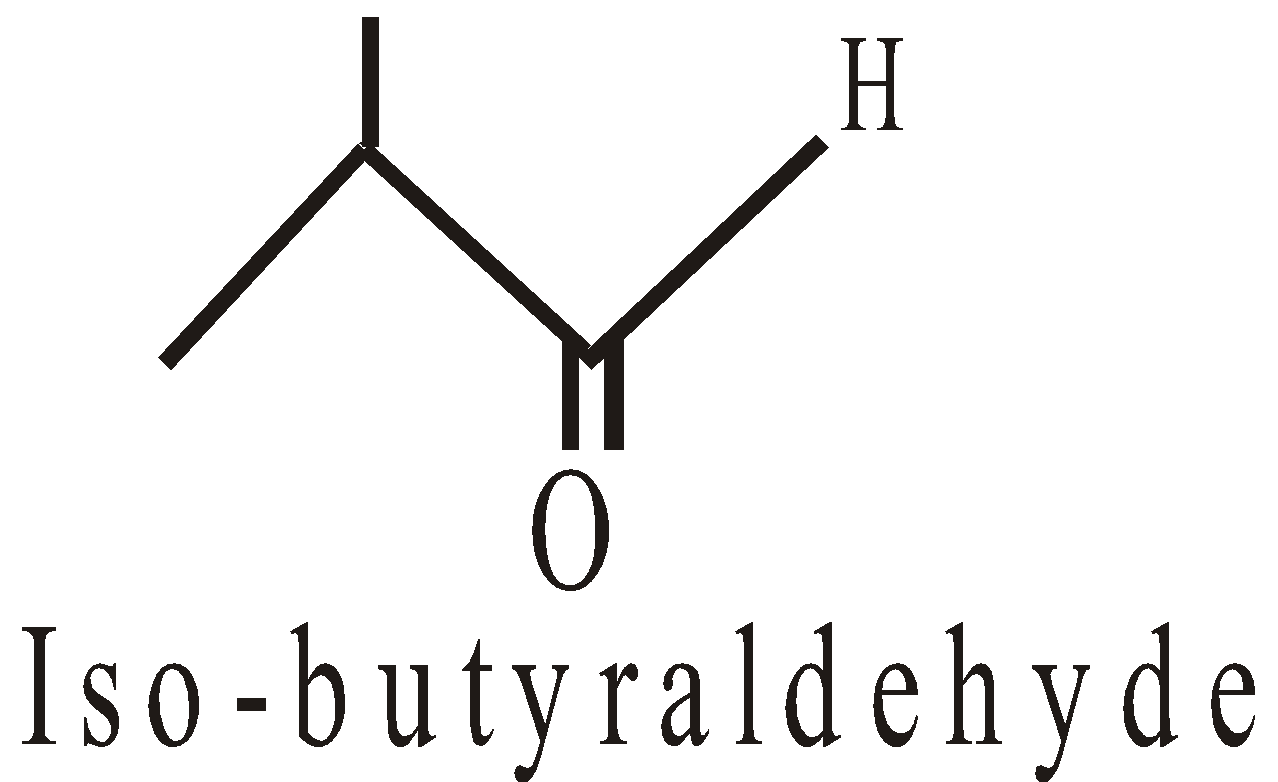
Functional isomerism


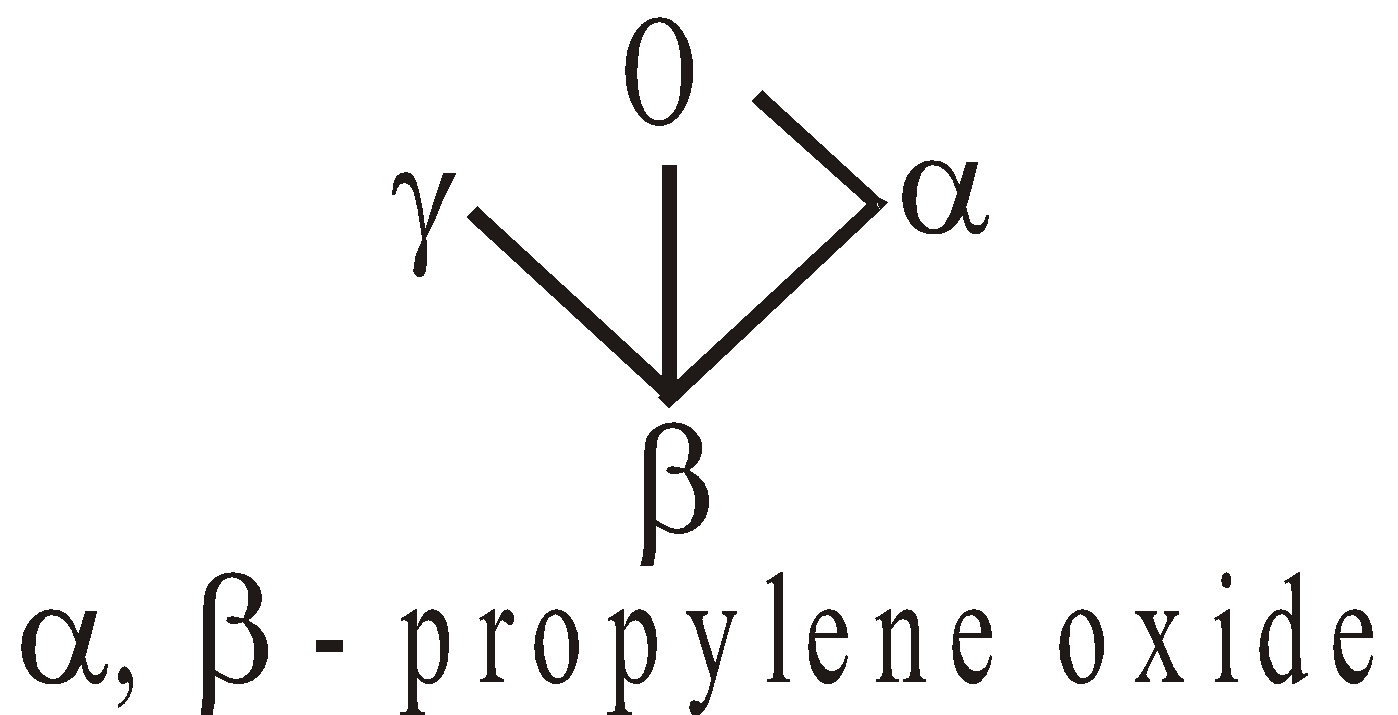
ISOMERISM IN KETONES
They exhibit three types of isomerism
Chain isomerism

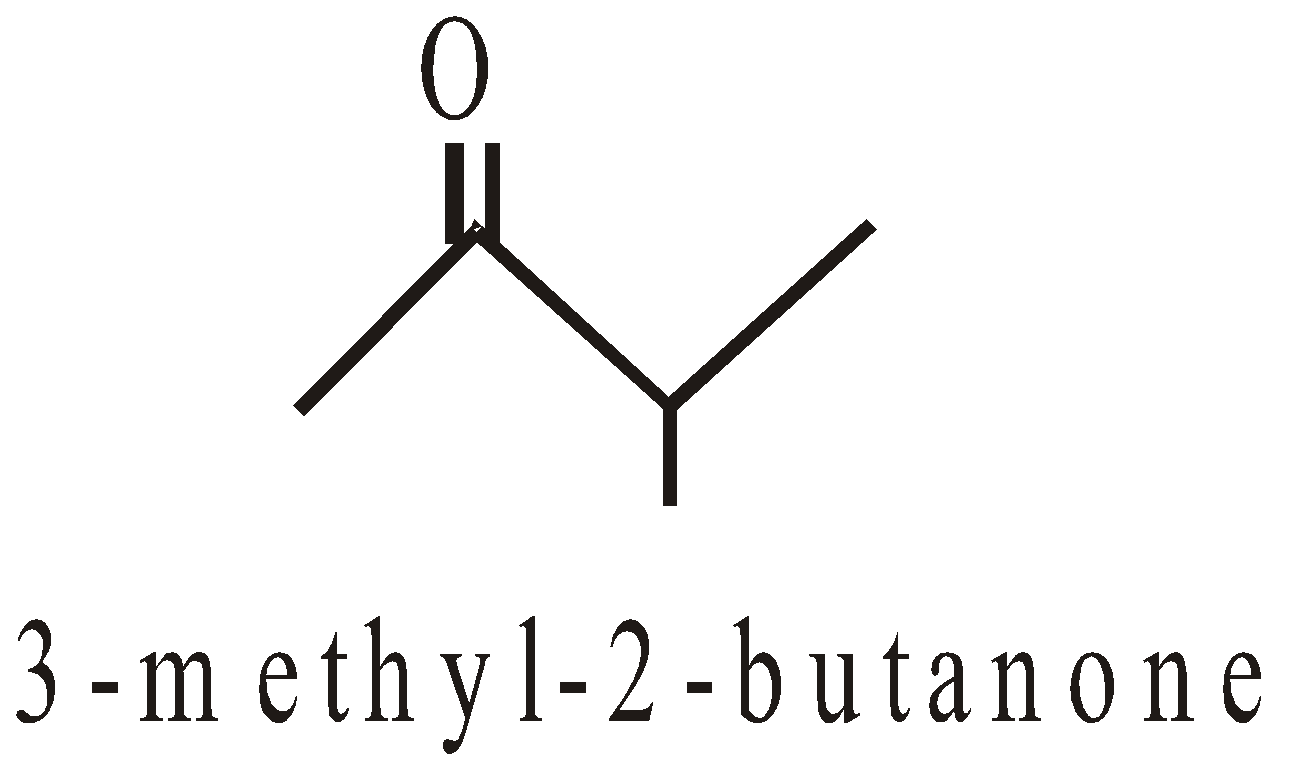 Functional isomerism
Functional isomerism

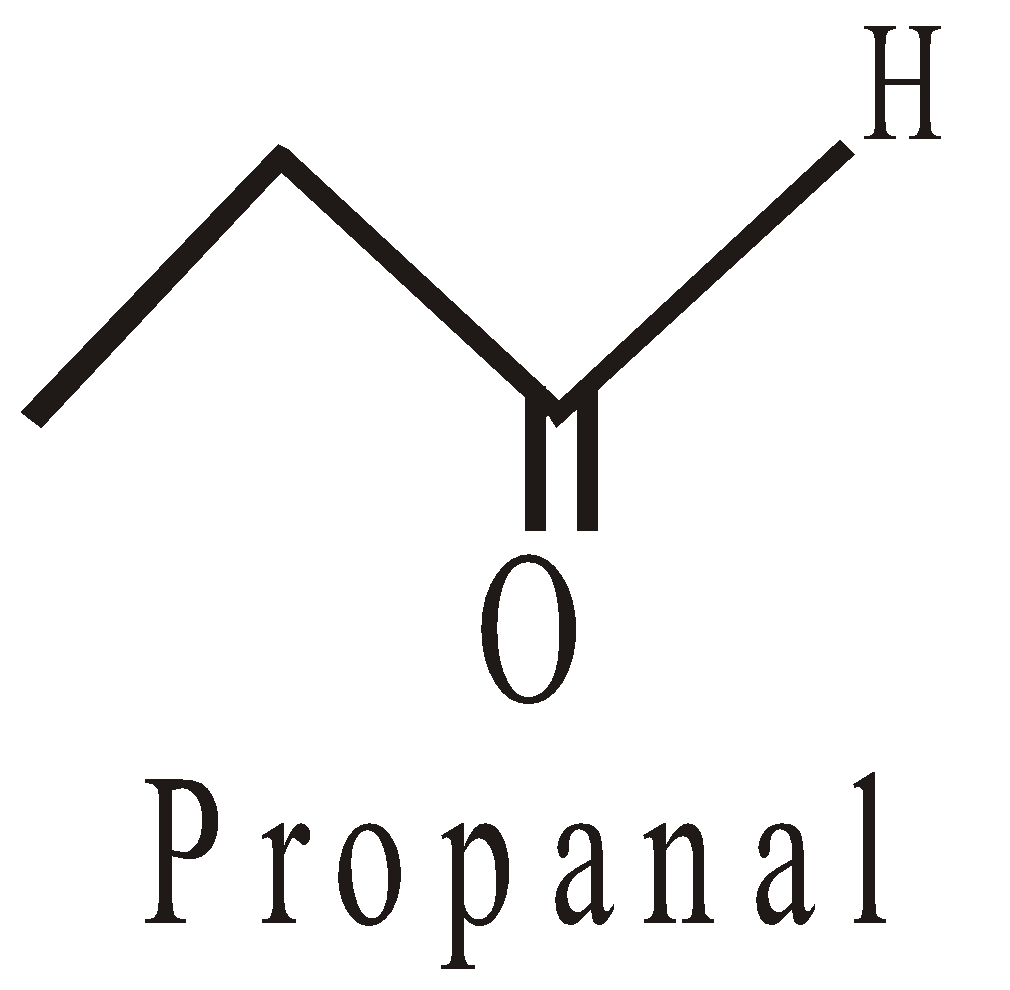
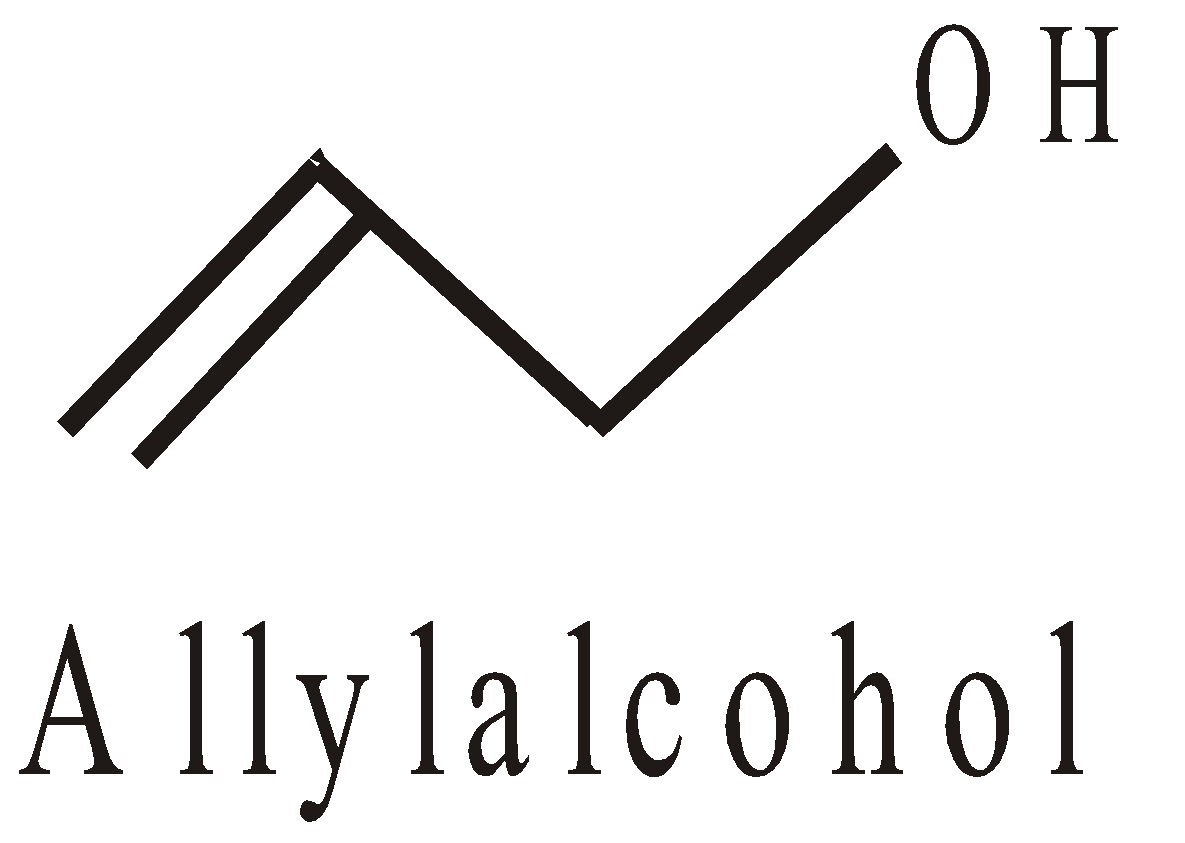 Position isomerism
Position isomerism


GENERAL METHODS OF PREPARATION OF ALDEHYDES
-
Controlled oxidation of primary alcohols
Oxidising agents K2Cr2O7/H2SO4 or KMnO4/H2SO4

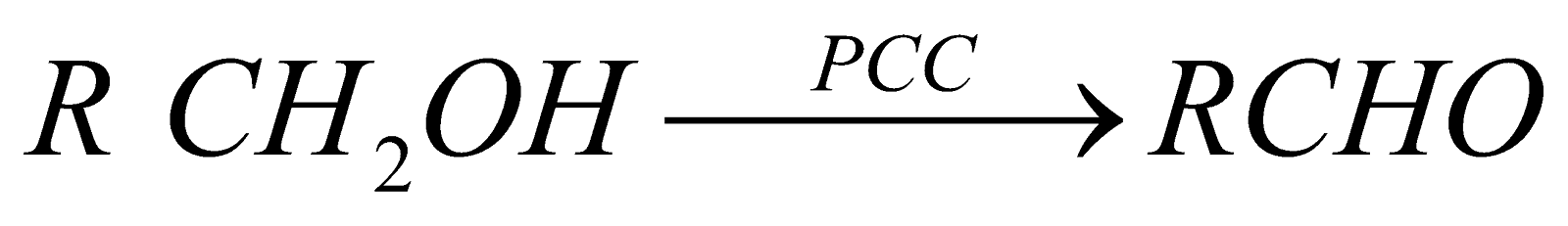 PCC is pyridinium chlorochromate known as Collin’s reagent and is specific for oxidation of 1º alcohol to aldehyde.
PCC is pyridinium chlorochromate known as Collin’s reagent and is specific for oxidation of 1º alcohol to aldehyde.
-
Dehydrogenation of primary alcohol
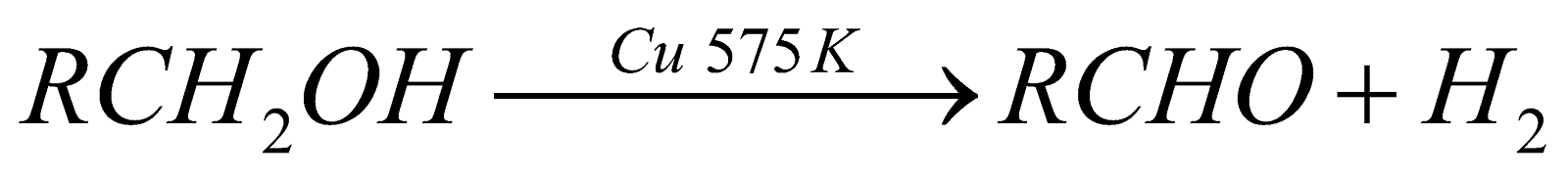
-
By Rosenmund reduction from acid chloride

-
Hydration of Alkynes (Kucherov reaction)
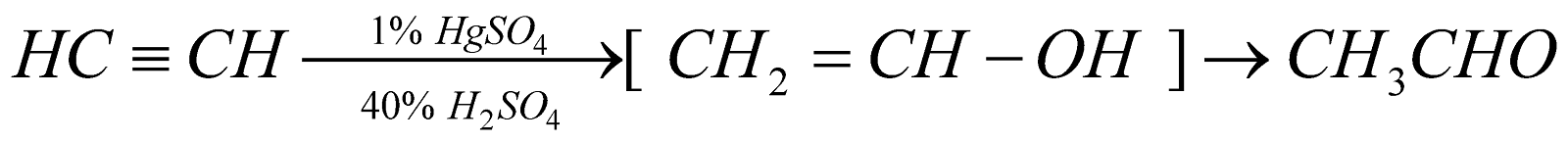
-
Reductive ozonolysis of alkenes
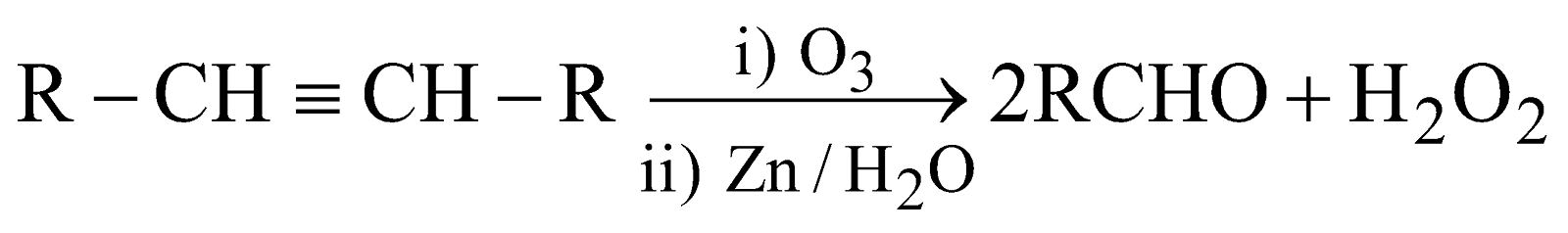
-
Distillation of Calcium salt of fatty acid with calcium formate
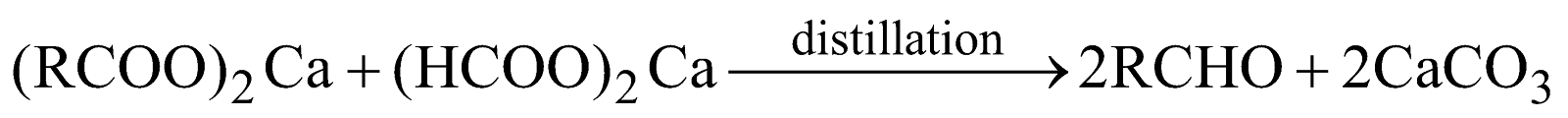
-
From acid
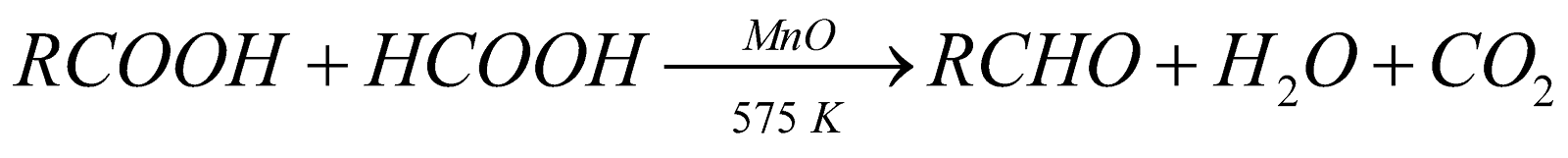

-
Waker’s process

-
Oxo process
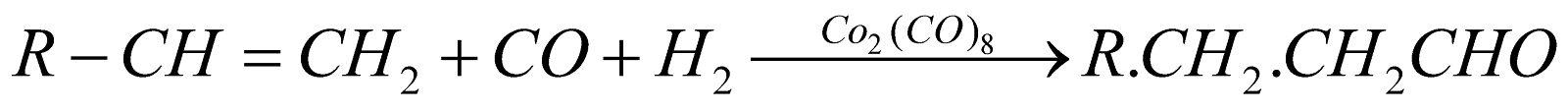
-
Stephen’s reduction of nitriles :
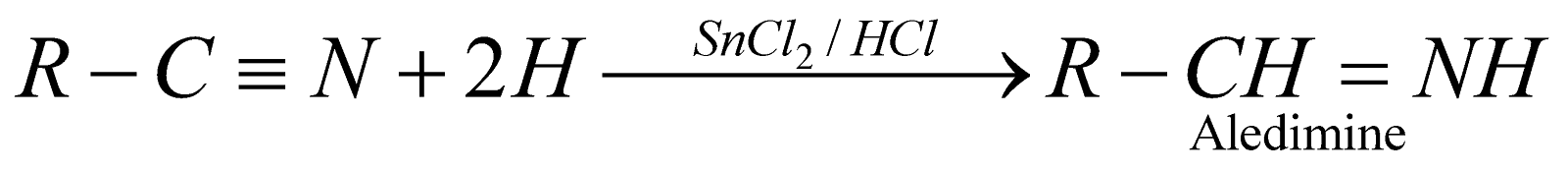
-
Hydrolysis of Geminal halides

-
From Glycols
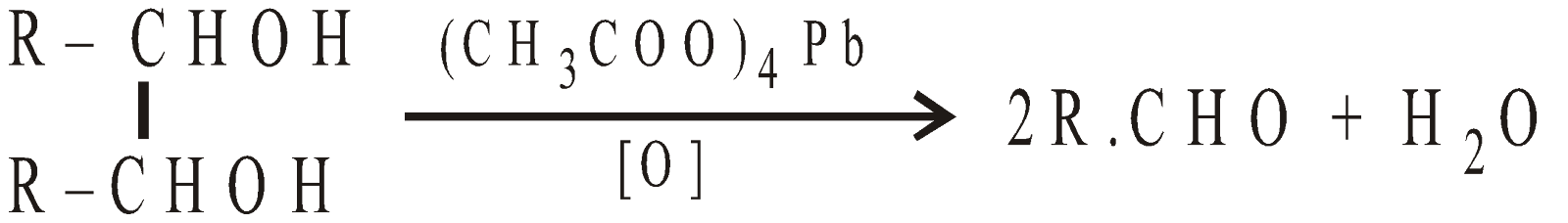
-
From Grignard’s reagent
-
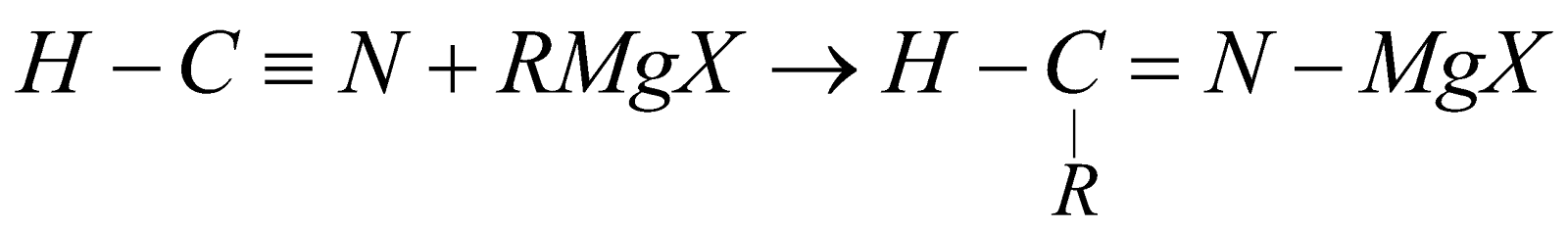
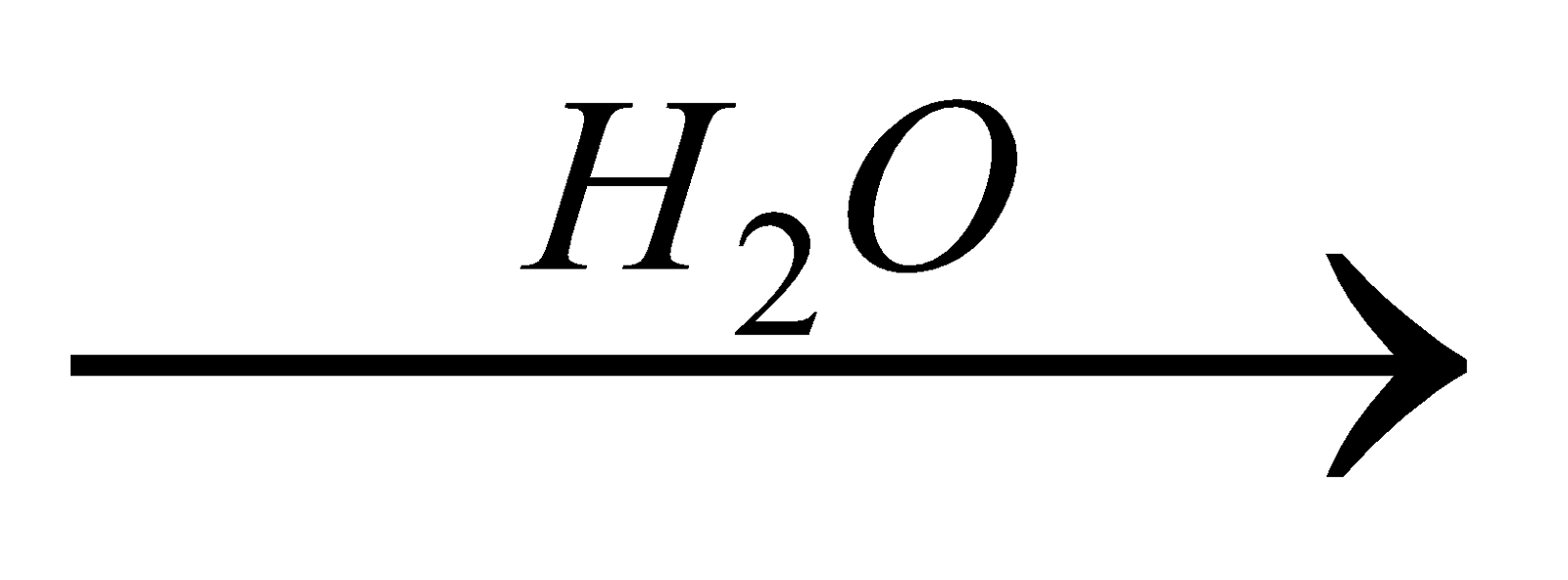
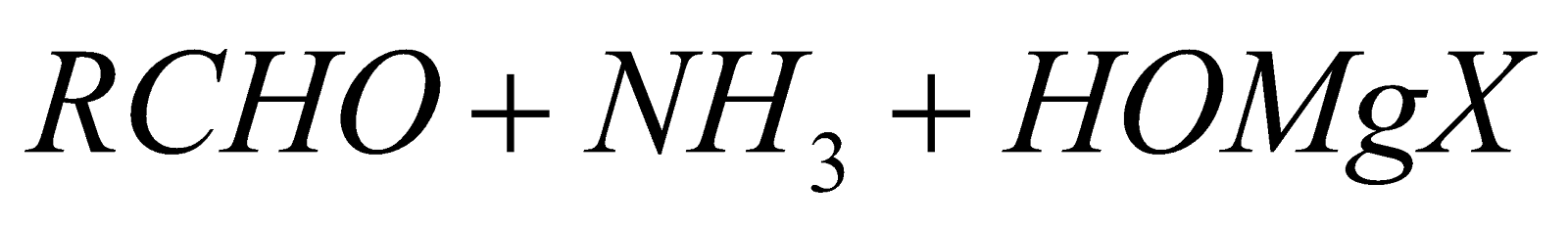
-
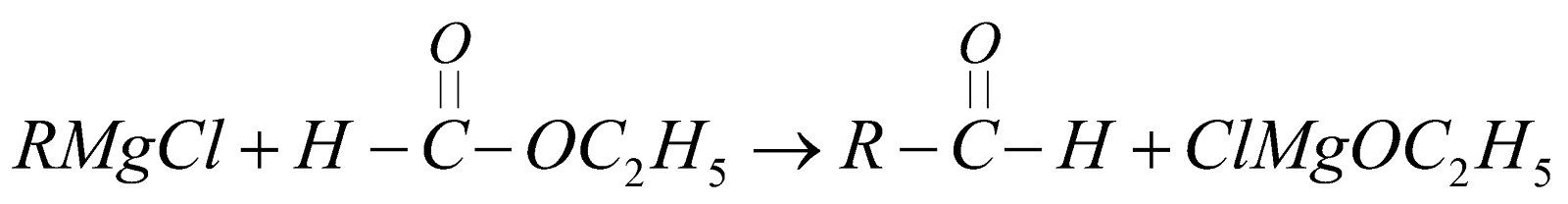
-
From acid chloride by the use of lithium t-butoxy aluminium hydride


GENERAL METHODS OF PREPARATION OF KETONES ONLY
-
Oxidation of 2º alcohol
Oxidising agent K2Cr2O7/H2SO4 or KMnO4/H2SO4
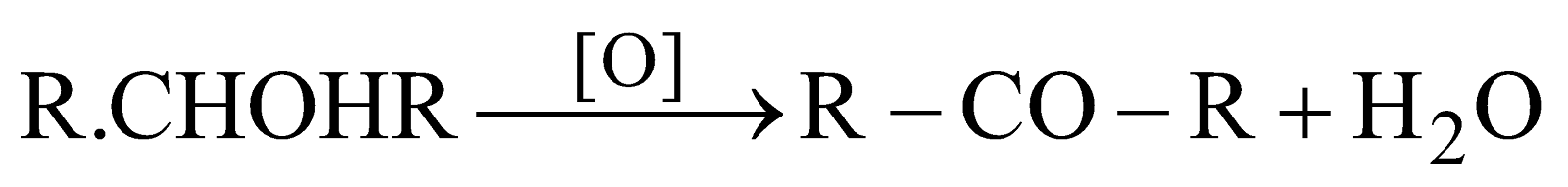
-
Dehydrogenation of 2º alcohols

-
Hydration of alkynes
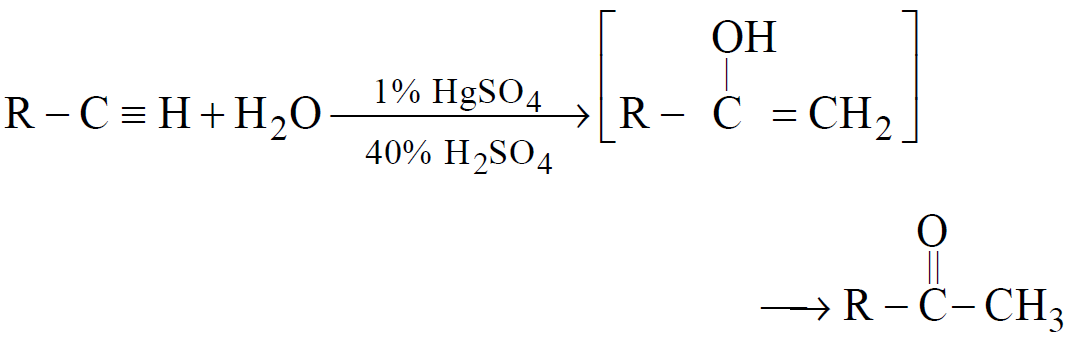
-
Reductive ozonolysis of alkenes

-
From Calcium salt of an acid

-
From acid

-
Hydrolysis of non-terminal gem.halide

-
From Grignard’s reagents
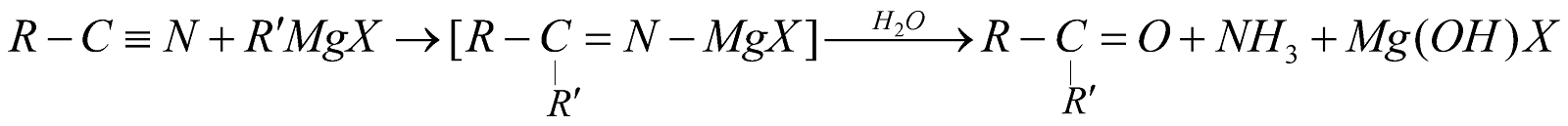
-
From acid chlorides
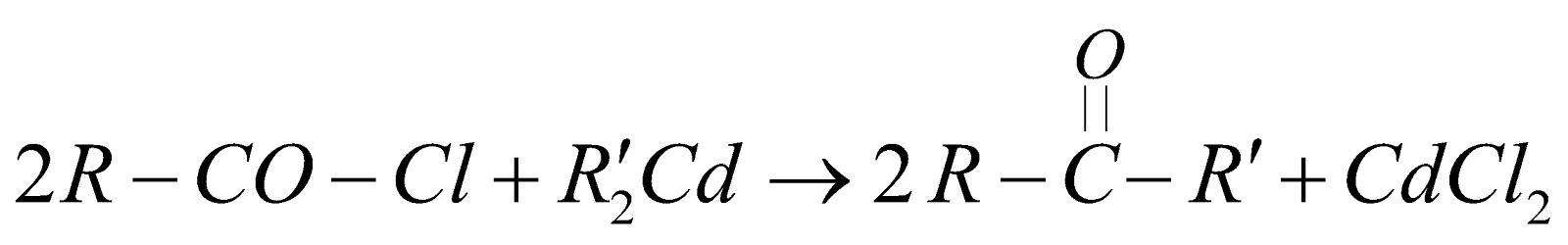
PHYSICAL PROPERTIES
-
Formaldehyde is a gas and its 40% aqueous solution was known as formalin but now it is 40% HCHO, 8% CH3OH, 52% H2O. They are polar in nature and have higher values of b.p. Lower members are soluble in water.
-
Lower aldehydes and ketones (C1 – C4) are soluble in water due to presence of H–bonding.


-
Reactivity
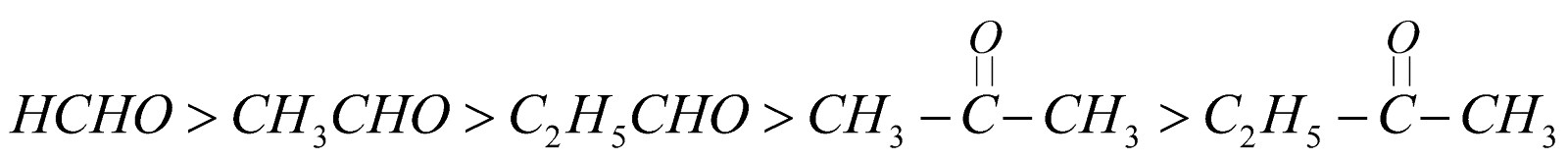
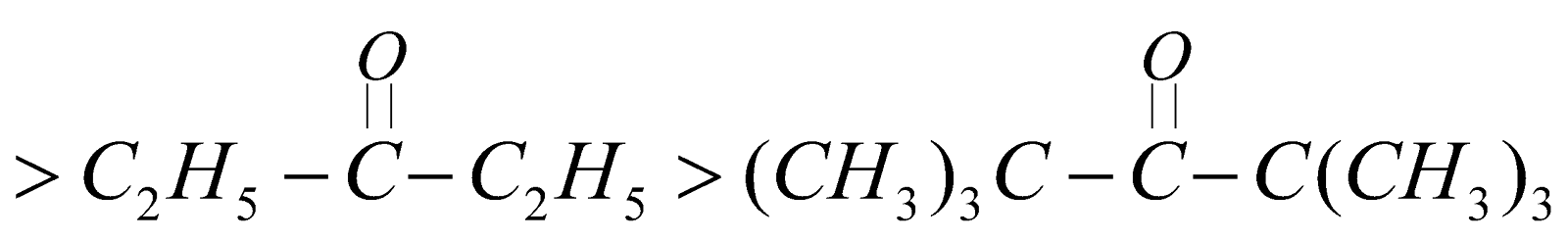 It is due to + I effect of alkyl groups which decreases the +ve charge on carbonyl carbon.
It is due to + I effect of alkyl groups which decreases the +ve charge on carbonyl carbon.
Steric hindrance : The bulky alkyl group hinder the approach of nucleophile.
a-hydrogen atom is acidic in nature due to Resonance

CHEMICAL PROPERTIES
ADDITION REACTIONS
Their addition reactions are known as nucleophilic addition reactions
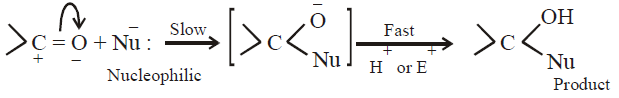

The followings reaction in different ways with NH3

 It is used as urinary antiseptic.
It is used as urinary antiseptic.

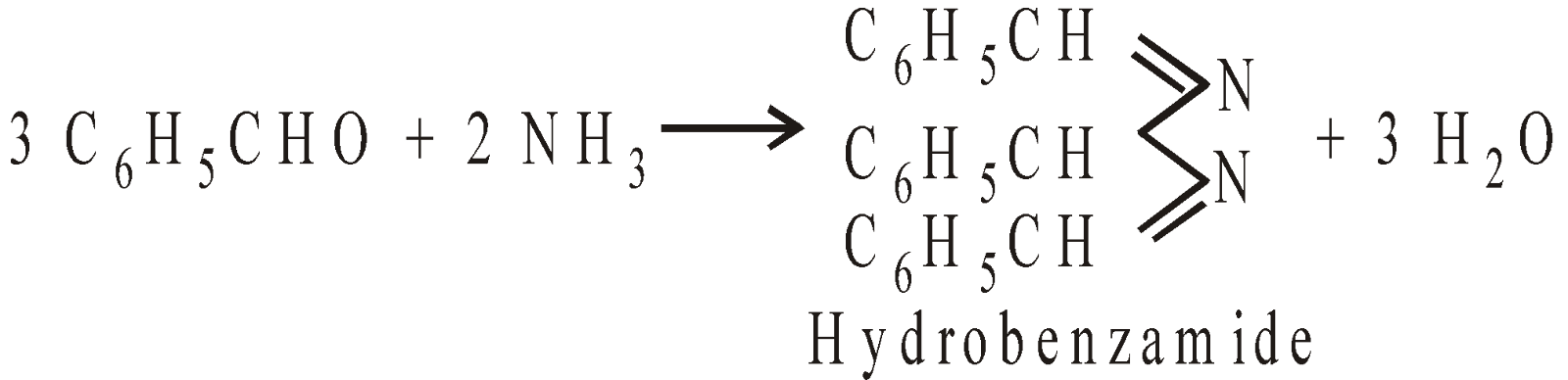
NUCLEOPHILIC ADDITION REACTIONS WITH ELIMINATION OF WATER MOLECULE
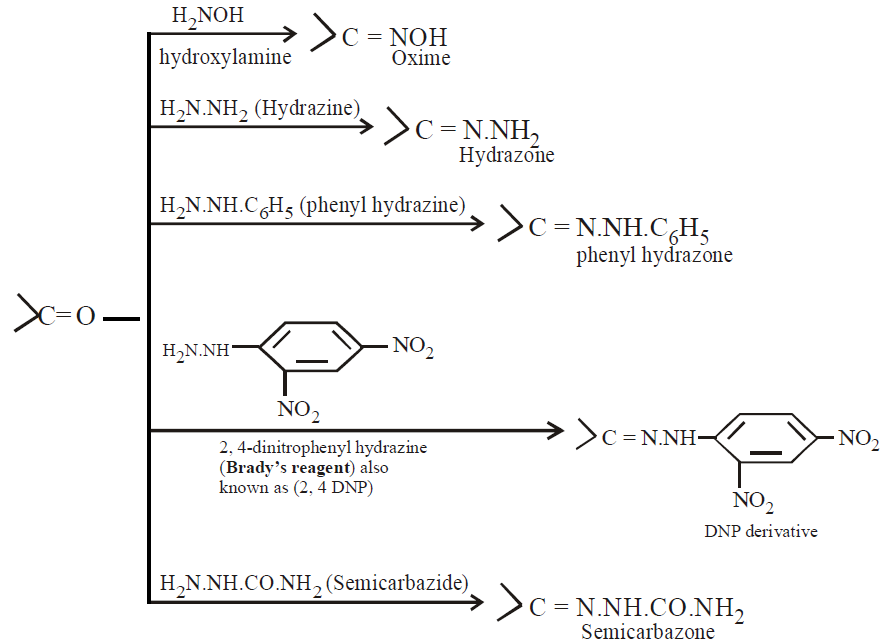 The control of pH is must for these reactions. The optimum value is around 3.5.
The control of pH is must for these reactions. The optimum value is around 3.5.
Control of pH during formation of ammonia derivatives :
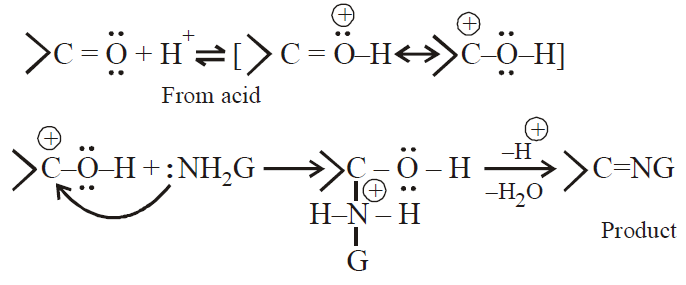 At low pH : H+ concentration is very high. The carbonyl compound and ammonia derivative, both protonated and latter cannot act as nucleophile .
At high pH : H+ concentration is too small. The protonation of carbonyl group will not occur and reaction will not occur smoothly.
Hence, optimum pH of the medium is around 3.5.
At low pH : H+ concentration is very high. The carbonyl compound and ammonia derivative, both protonated and latter cannot act as nucleophile .
At high pH : H+ concentration is too small. The protonation of carbonyl group will not occur and reaction will not occur smoothly.
Hence, optimum pH of the medium is around 3.5.
OXIDATION
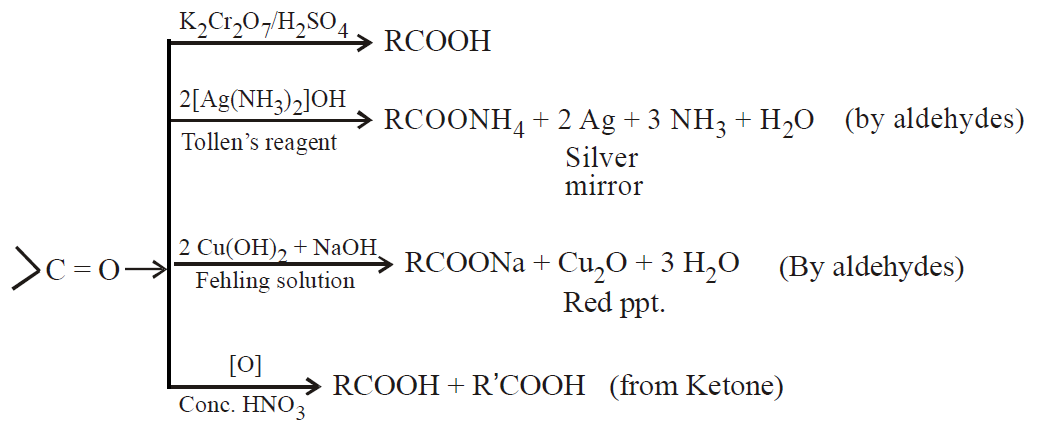 Ketones are oxidised by strong oxidising agents such as Conc. HNO3, K2Cr2O7/H2SO4, KMnO4/H2SO4
Ketones are oxidised by strong oxidising agents such as Conc. HNO3, K2Cr2O7/H2SO4, KMnO4/H2SO4
OXIDATION WITH SeO2
The CH3 group adjacent to  is oxidised to – CHO and >CH2 group is oxidised to
is oxidised to – CHO and >CH2 group is oxidised to
>C = O group.


POPOFF’S RULE
During oxidation of unsymmetrical ketone the carbonyl group is retained by smaller alkyl group.
 (major products)
Jone’s reagent : Acidified K2Cr2O7 i.e. chromic acid and sulphuric acid mixture
(major products)
Jone’s reagent : Acidified K2Cr2O7 i.e. chromic acid and sulphuric acid mixture
HALOFORM REACTION
Oxidation of acetaldehyde or methyl ketones with Sodium Hypohalite (NaOX) or (X2 + NaOH) gives haloform CHX3. The reaction is of practical value to identify these compounds by forming CHI3.
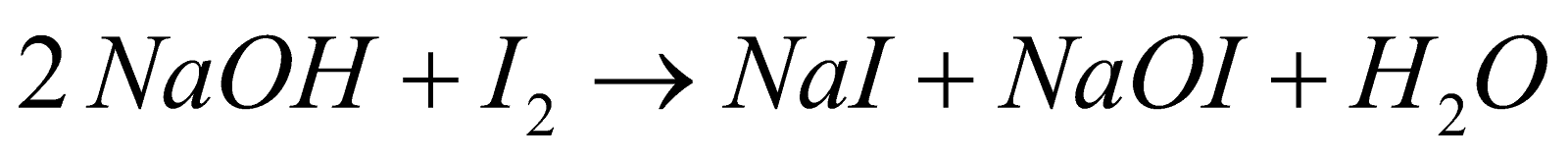

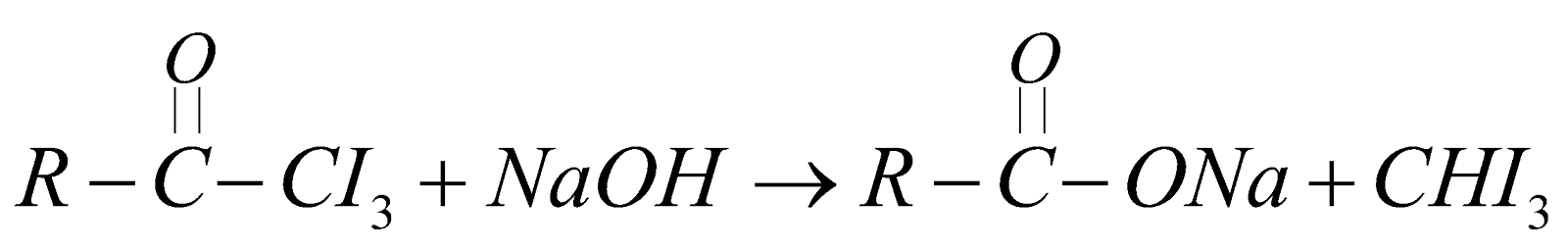
BAYER-VILLIGER OXIDATION
Oxidation of aliphatic Ketones by organic per acids, e.g. perbenzoic acid, peracetic acid or monoperthalic acid to form esters or their hydrolysed products
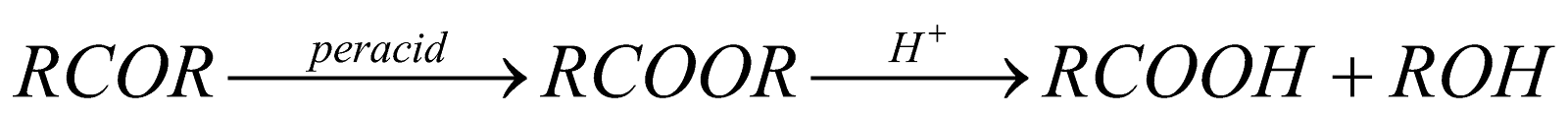
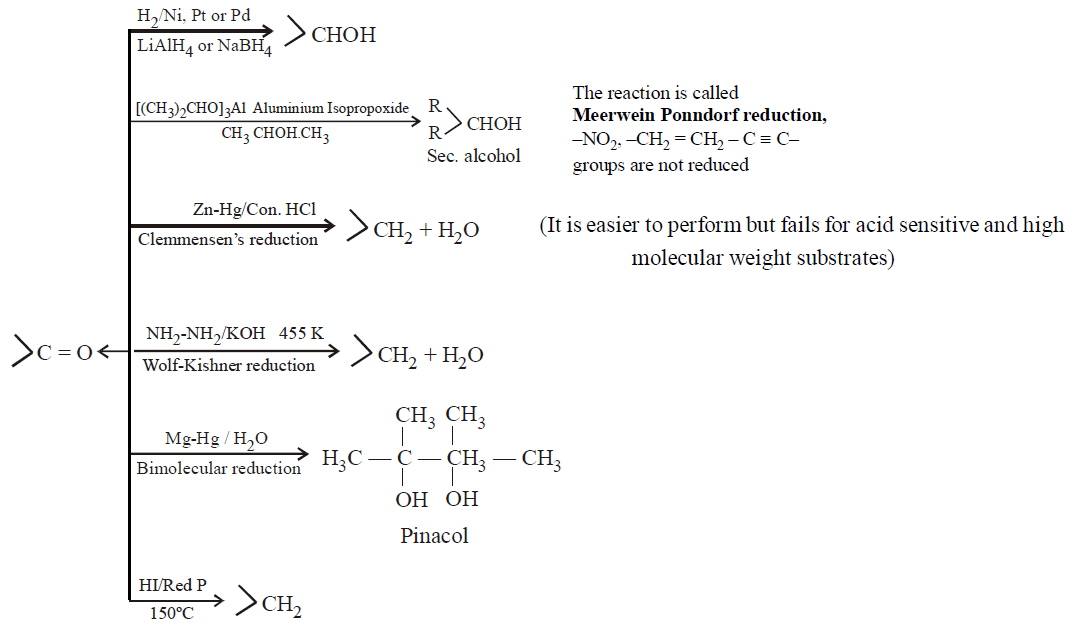
REDUCTION
CONDENSATION REACTIONS
ALDOL CONDENSATION
Aldehydes and Ketones having at least one a-hydrogen atom in presence of dil. alkali give b- hydroxy aldehyde or b-hydroxy ketone, which on heating gives a,b-unsaturated carbonyl compound.
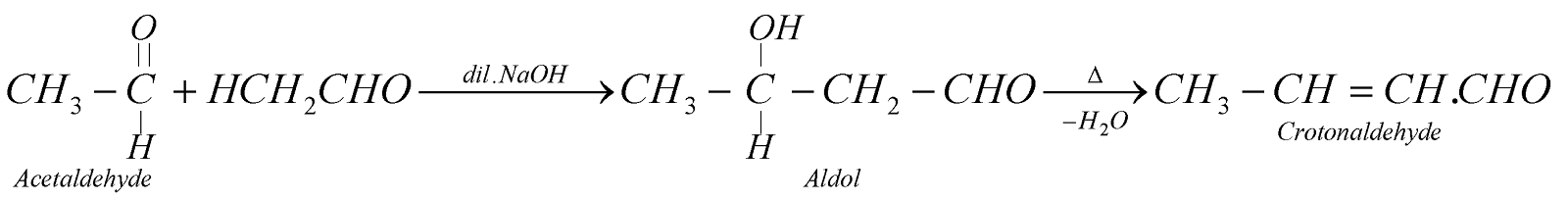
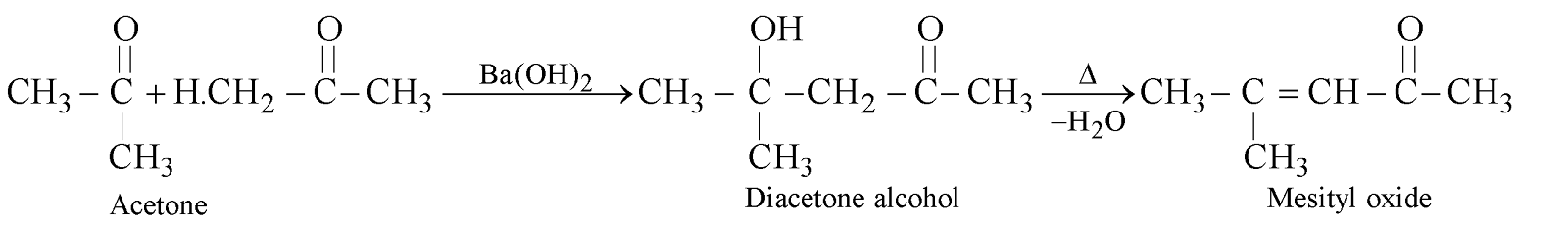
CROSSED ALDOL CONDENSATION


INTRAMOLECULAR ALDOL CONDENSATION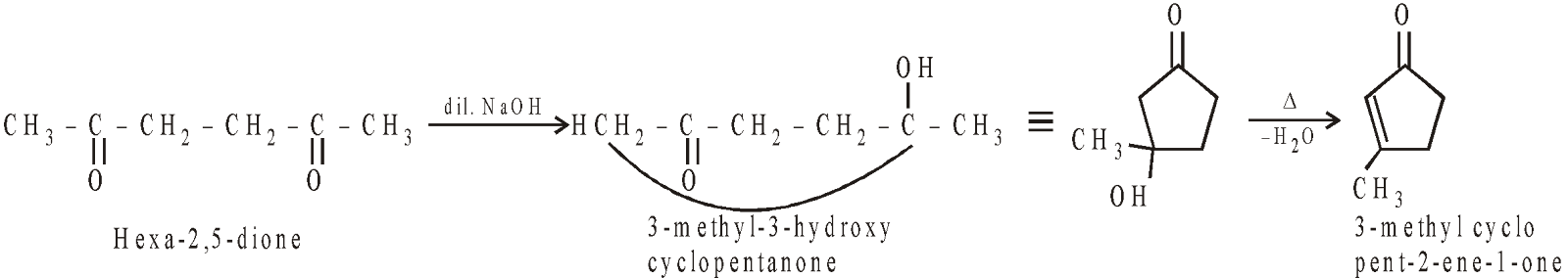
CANNIZZARO’S REACTION
Aldehydes containing no a-hydrogen atom on warming with 50% NaOH or KOH undergo disproportionation i.e. self oxidation - reduction known as cannizzaro’s reaction.
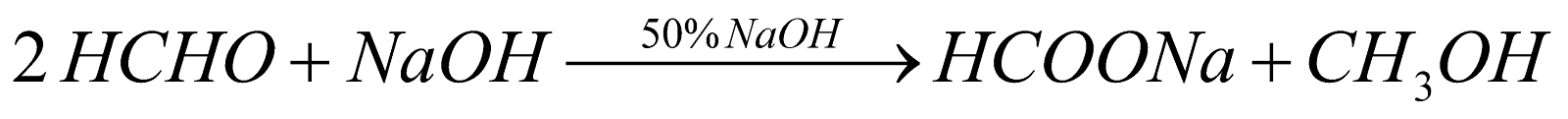

 * 2-methyl propanal (CH3)2CH.CHO has a-hydrogen atom but gives Cannizzaro’s reaction.
* 2-methyl propanal (CH3)2CH.CHO has a-hydrogen atom but gives Cannizzaro’s reaction.
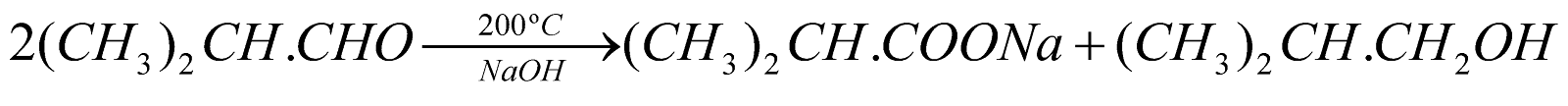 Aldehydes containing a-H atoms on heating with conc. alkali give brown resinous mass by undergoing repeated aldol condensation.
Aldehydes containing a-H atoms on heating with conc. alkali give brown resinous mass by undergoing repeated aldol condensation.
CROSSED CANNIZZARO’S REACTION

TISHCHENKO REACTION
Aldehydes containing a-hydrogen atom with aluminium ethoxide give esters.
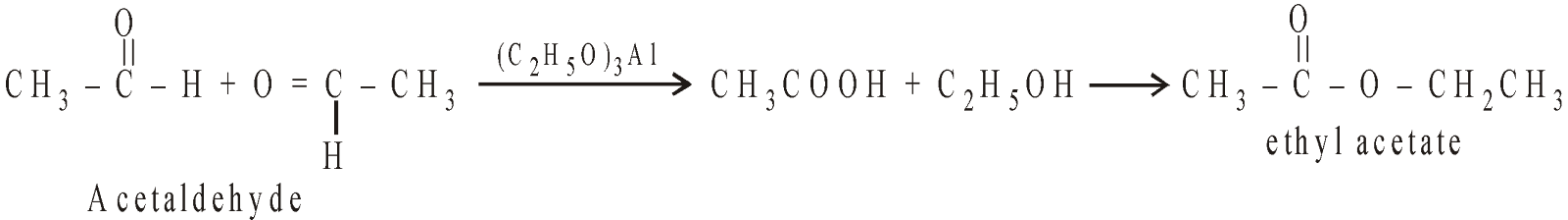
REFORMATSKY REACTION
It is the reaction between an a-bromo acid ester and a carbonyl compound (aldehyde or ketone) in the presence of zinc to form a b-hydroxy ester.

BECKMANN’S REARRANGEMENT
It is rearrangement of keto oxime to N-substituted acid amide in presence of Conc. H2SO4, PPA, SOCl2, PCl5 etc.

CONDENSATION PRODUCTS OF ACETONE

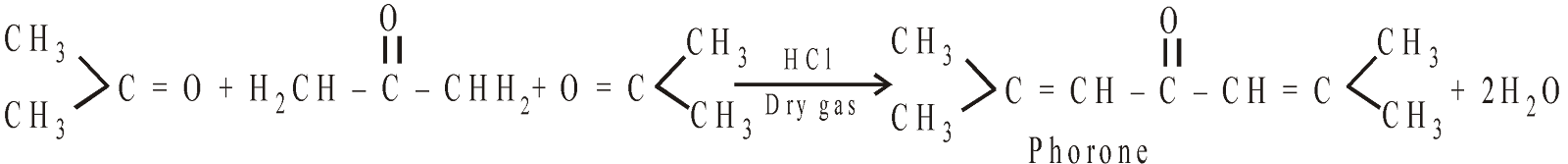
 Above reactions are not aldol condensations.
Above reactions are not aldol condensations.
POLYMERS OF FORMALDEHYDES
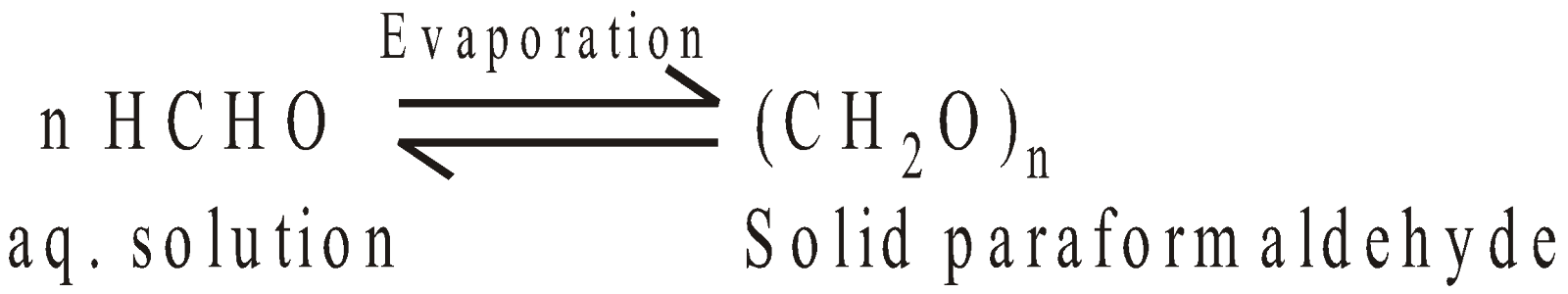
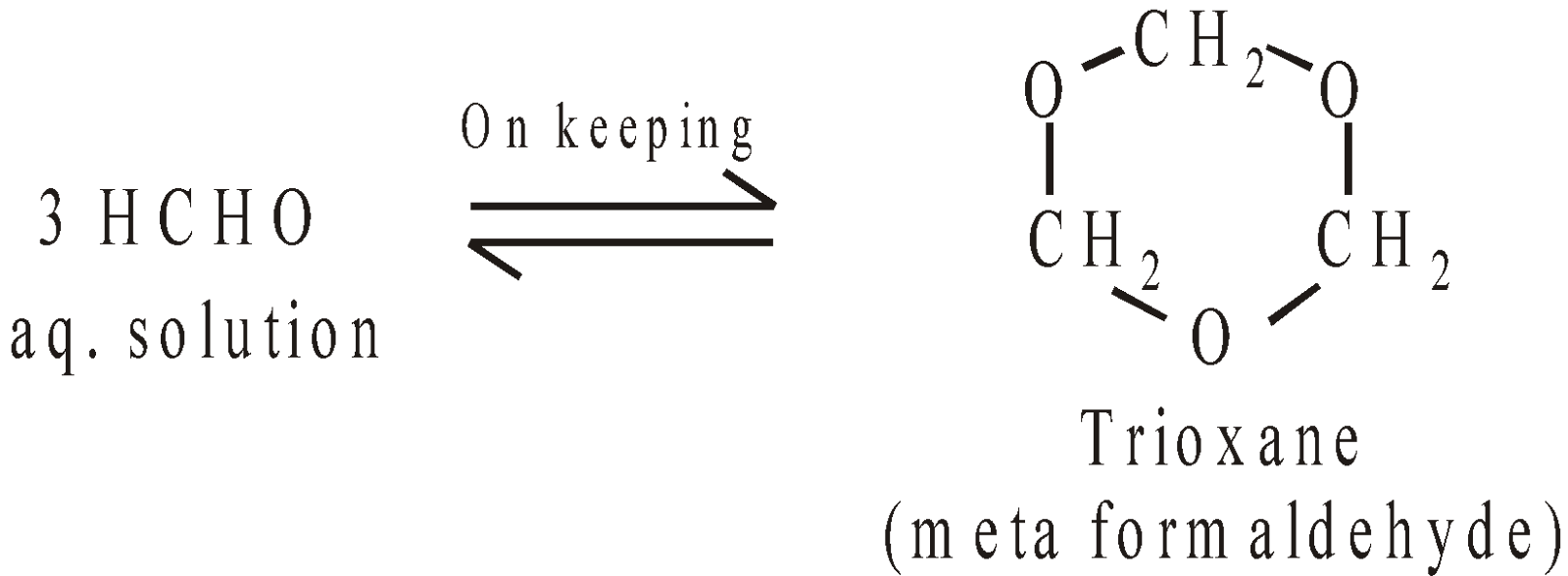
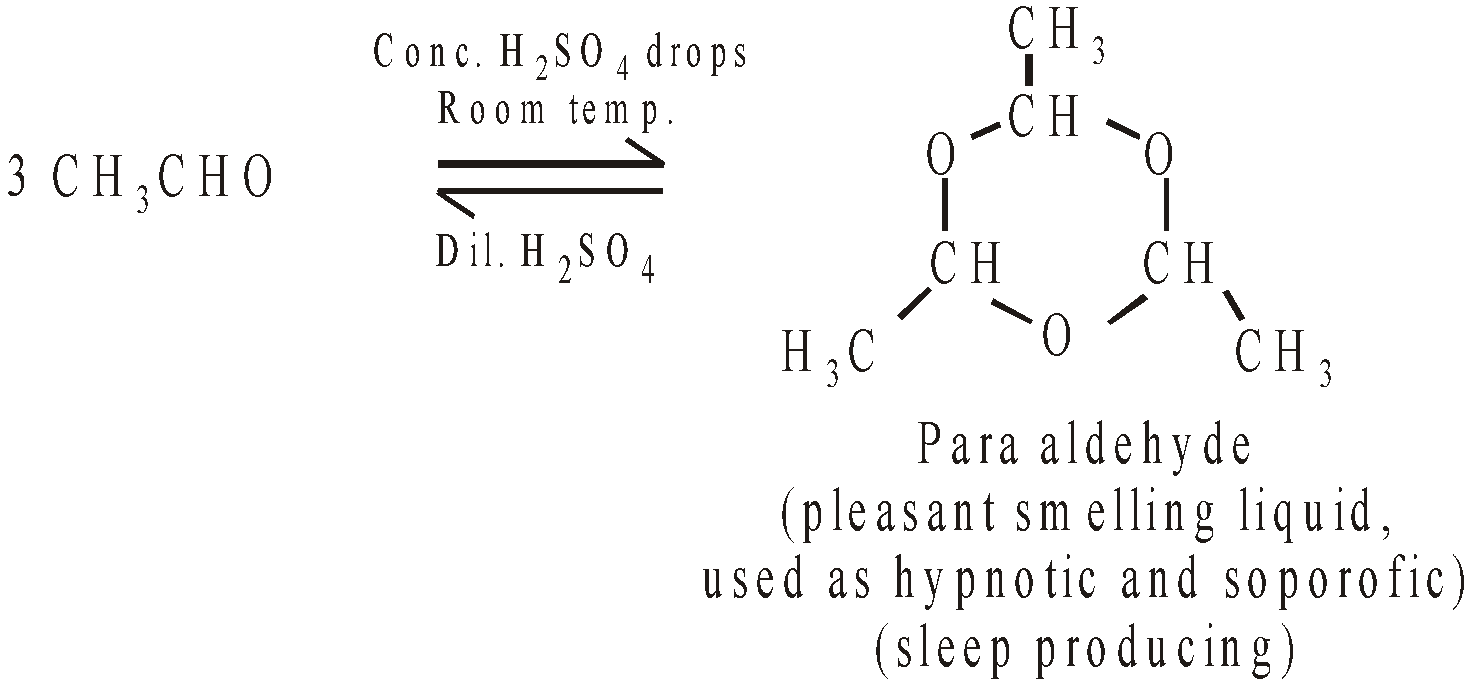
POLYMERS OF ACETALDEHYDE

BENZALDEHYDE (AROMATIC ALDEHYDES)
PREPARATION
-
Etard’s oxidation

-
Oxidation of toluene
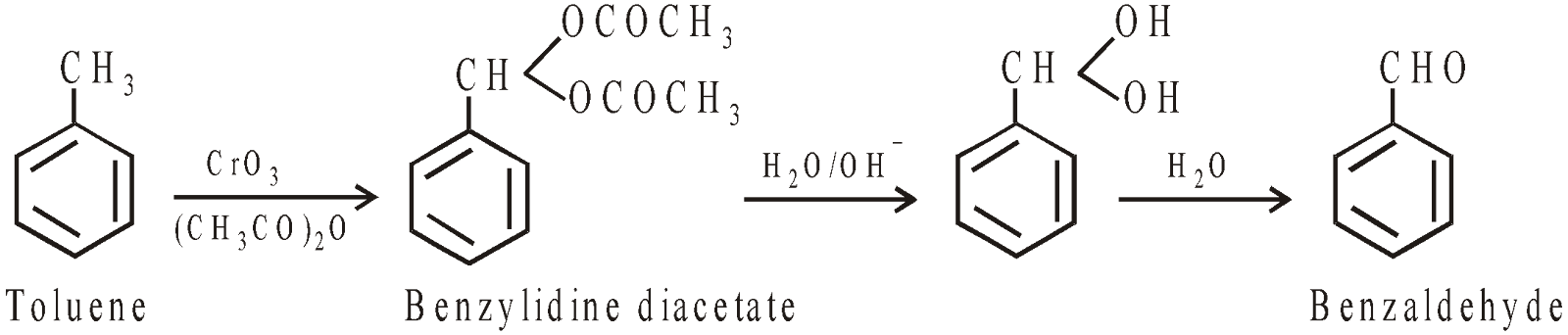
-
Gattermann Koch Synthesis
 (Combination of CO + HCl act as formyl chloride HCOCl)
It is an electrophilic substitution reaction, the electrophile
(Combination of CO + HCl act as formyl chloride HCOCl)
It is an electrophilic substitution reaction, the electrophile 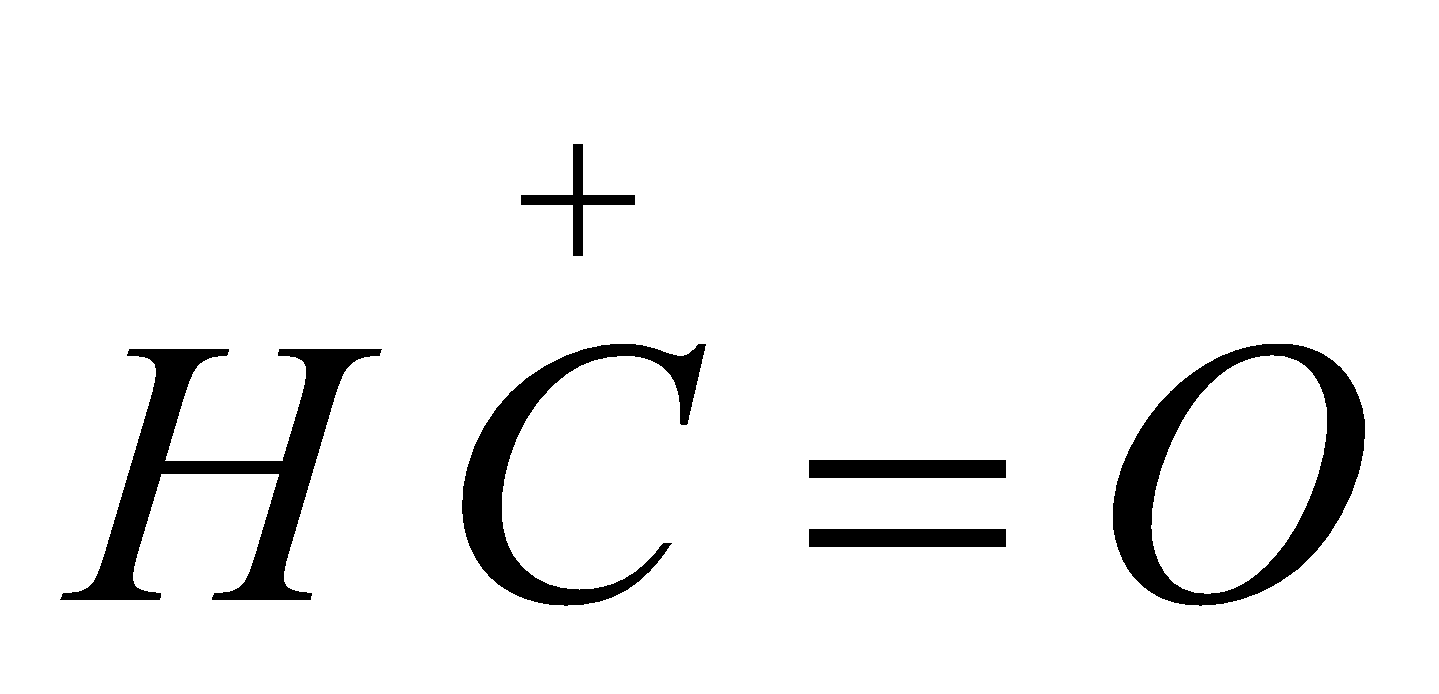 is formylium ion.
is formylium ion.
-
Gattermann aldehyde synthesis
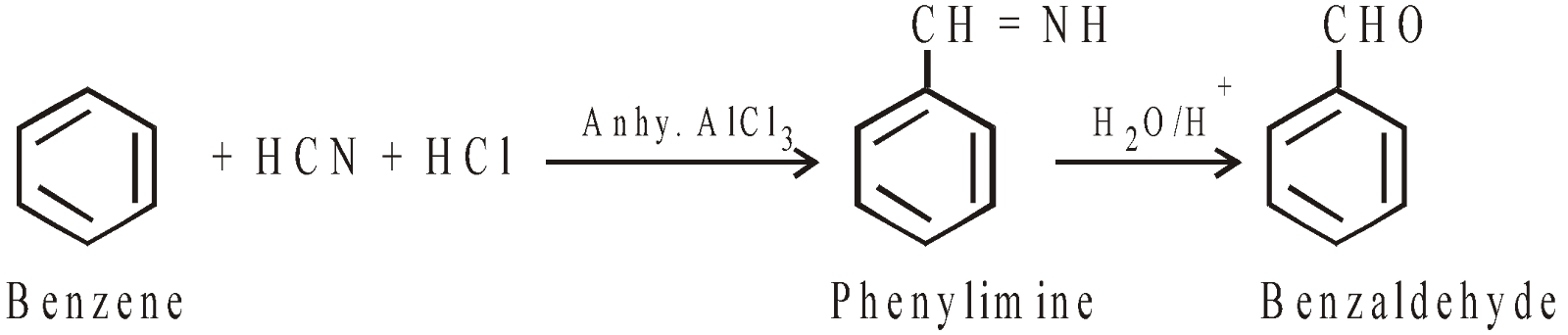
-
From benzyl chloride (Lab method)

-
Rosenmund reaction

-
Vilsmeyer reaction

-
From Grignard’s reaction
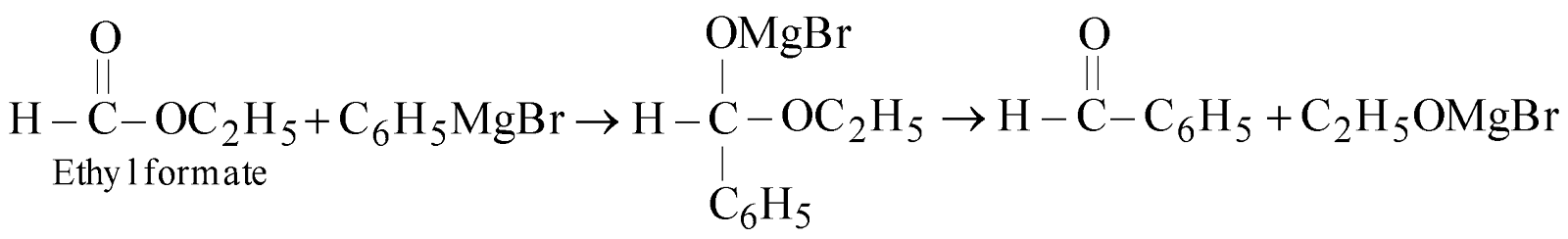
-
From Benzal chloride (manufacture)
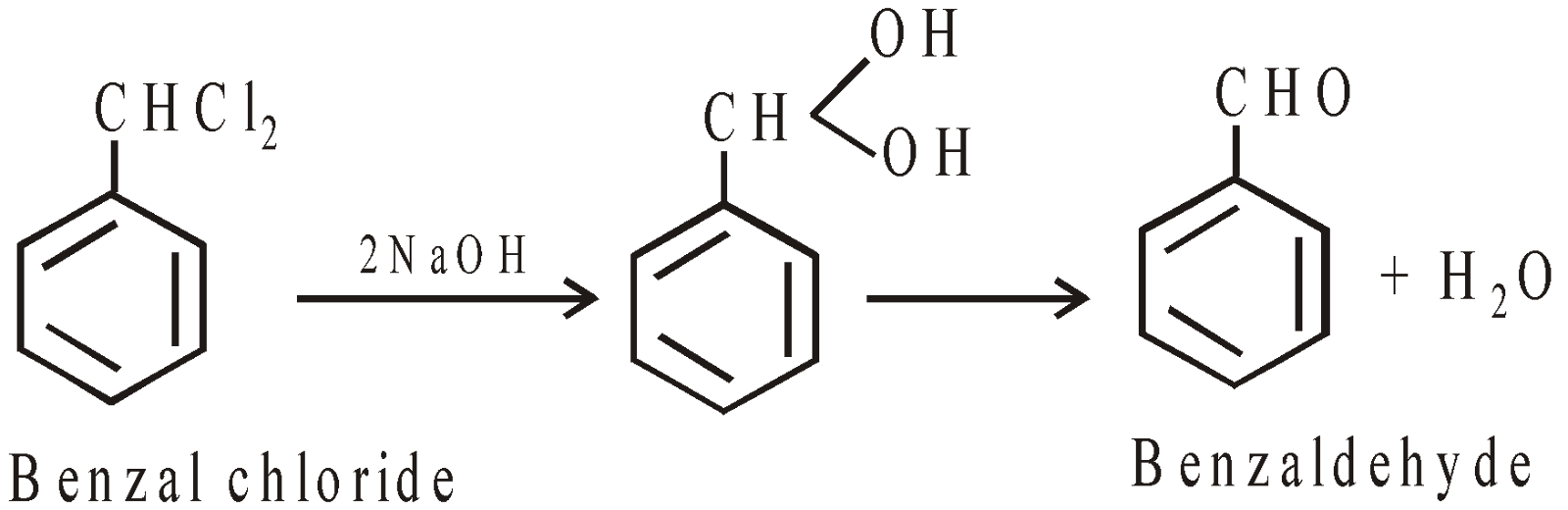
-
Partial oxidation of toluene (manufacture)
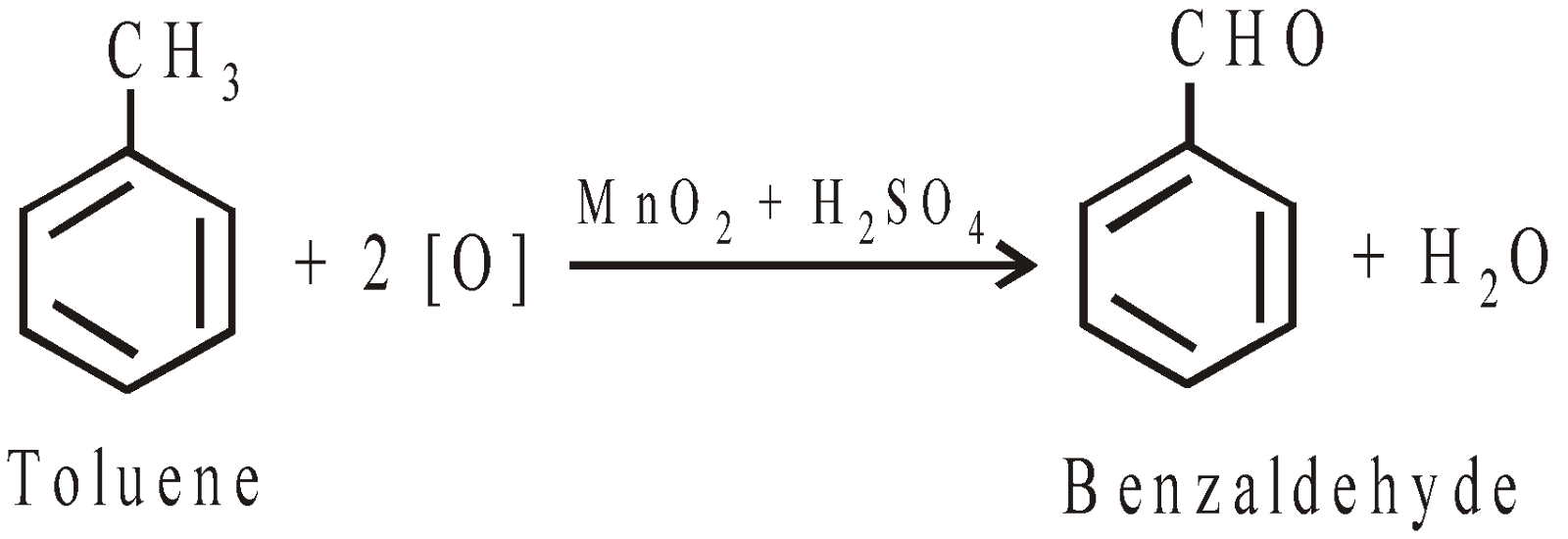
PHYSICAL PROPERTIES
Colourless, highly refractive liquid b.p. 443K. It has smell of bitter almonds, slightly soluble in water. Steam volatile and poisonous.
CHEMICAL PROPERTIES
REACTION OF CHO GROUP GIVEN BY AROMATIC ALDEHYDES
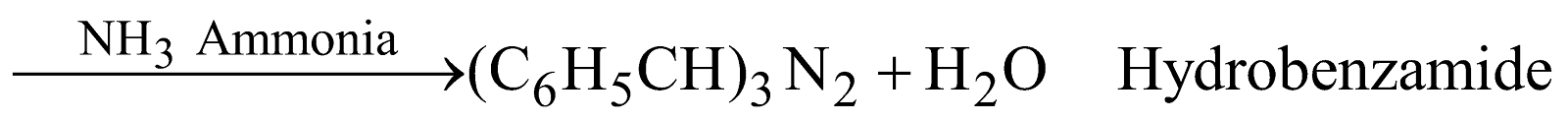
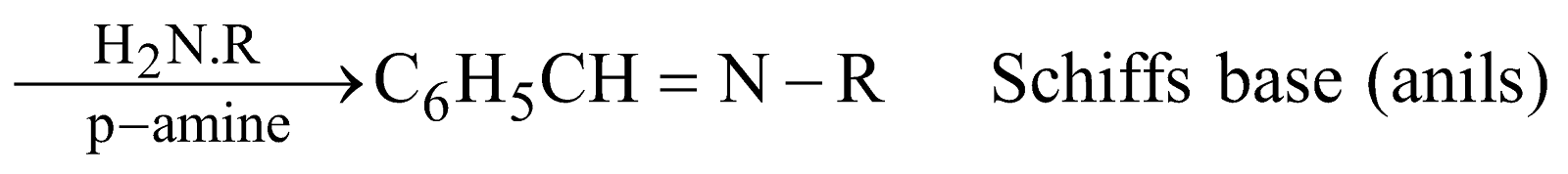

REACTIONS DUE TO BENZENE NUCLEUS
CHO group is meta directing with deactivation of benzene nucleus
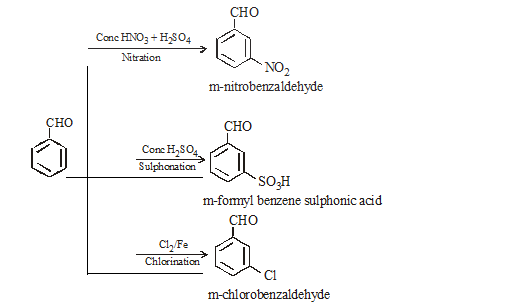
Benzaldehyde reduces Tollen’s reagent but not Fehling solution.
USES
-
As flavouring agent
-
In perfumes
-
Manufacture of triphenylmethane dyes
ACETOPHENONE (AROMATIC KETONES)
PREPARATION
-
By Friedel Craft’s acylation

-
From benzaldehyde

-
Manufacture

PHYSICAL PROPERTIES
It is colourless crystalline compound m.p. 20ºC, b.p. 202ºC.
At room temperature it is coloured liquid.
CHEMICAL PROPERTIES
-
Reactions due to
 . It gives almost all reactions due >C = O group.
. It gives almost all reactions due >C = O group.
-
Reactions due to benzene nucleus : –COCH3 is meta directing in nature. Hence electrophilic substitution reactions give meta derivative.
-
It give iodoform test.
-
Some important reactions are :
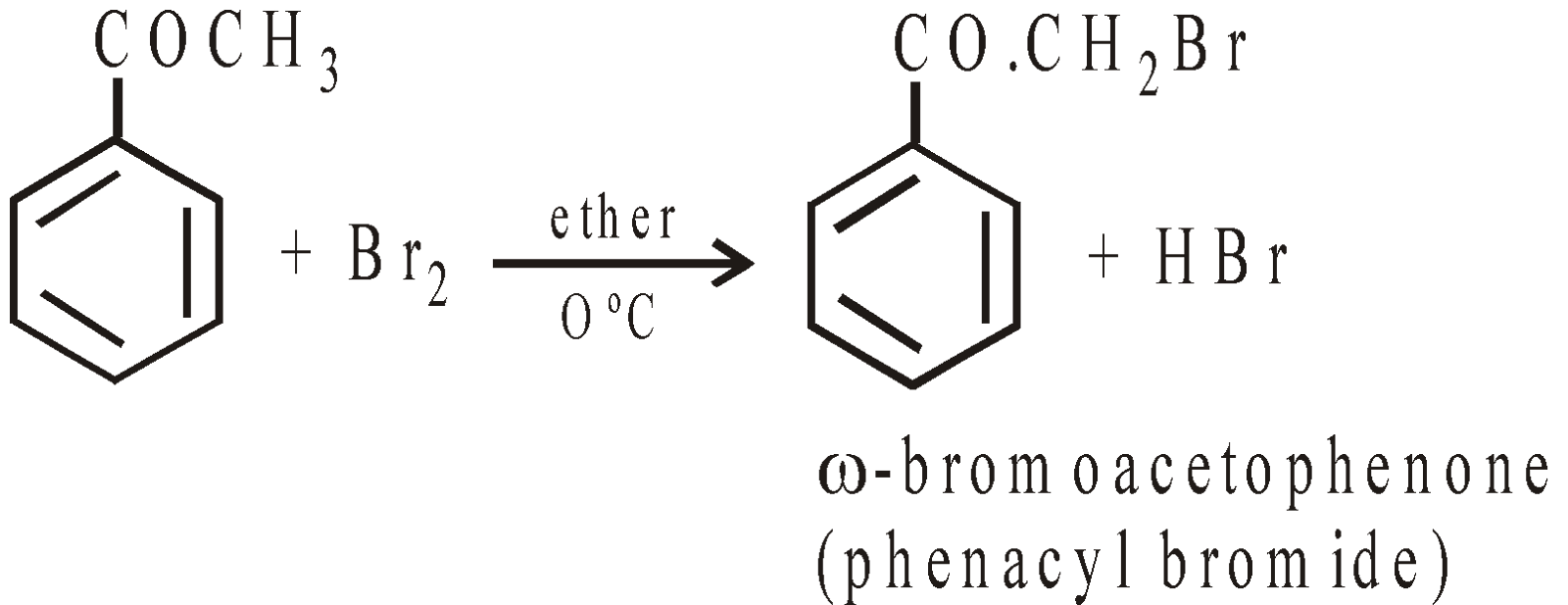 Its vapour attack nose, throat and lungs. It is used as tear gas.
Its vapour attack nose, throat and lungs. It is used as tear gas.

USES
It is used as hypnotic in medicine.
TEST FOR ALDEHYDE
-
Tollen’s reagent (ammoniacal silver nitrate). All aldehydes give silver mirror.

-
Fehling Solution (alkaline solution of Cu2+ complexed with Sodium potassium tartrate blue colour). All aldehydes give red colour except benzaldehyde.
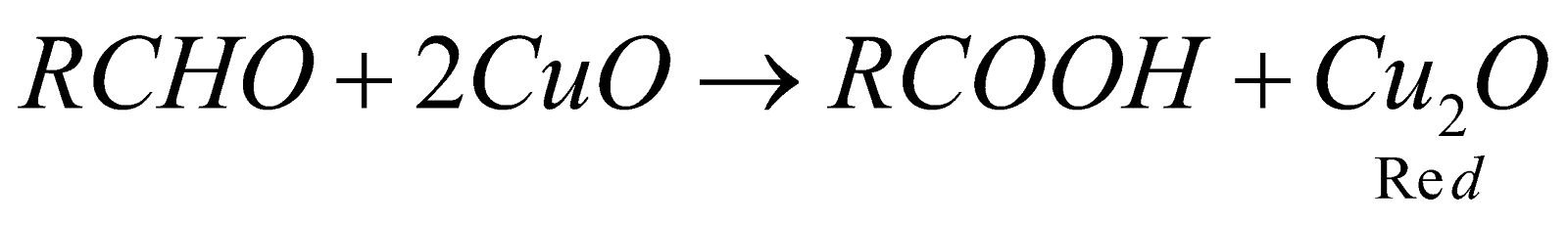
-
Benedict’s solution (Copper sulphate, sodium citrate and sodium carbonate solution). Aldehydes give reddish brown ppt.
-
Schiff’s reagent (dilute solution of p-rosaniline hydrochloride decolourised with SO2 or H2SO4). Aldehydes give pink colour.
Acetone respond to this test slowly and o-hydroxy benzaldehyde does not give pink colour with schiff’s reagent
Note : Formic acid, tartaric acid, a-hydroxy ketones, glucose, fructose reduce Tollen reagent and Fehling solution.
Oxidising agents K2Cr2O7/H2SO4 or KMnO4/H2SO4
Oxidising agent K2Cr2O7/H2SO4 or KMnO4/H2SO4
The CH3 group adjacent to
>C = O group.
During oxidation of unsymmetrical ketone the carbonyl group is retained by smaller alkyl group.
REACTIONS DUE TO BENZENE NUCLEUS
Benzaldehyde reduces Tollen’s reagent but not Fehling solution.
CARBOXYLIC ACIDS AND THEIR DERIVATIVES
CLASSIFICATION
- Monocarboxylic acids: containing one -COOH group
- dicarboxylic acids: containing two -COOH groups and so on
- Fatty acids: Aliphatic monocarboxylic acids are commonly called fatty acids because higher members are obtained by the hydrolysis of oils and fats.
NOMENCLATURE
They are named as Alkanoic acids eg.
ISOMERISM
eg. C6H12O2 represent
FUNCTIONAL ISOMERISM
GENERAL METHODS OF PREPARATION
- Oxidation of 1º alcohols and aldehydes with acid K2Cr2O7 or KMnO4
- Oxidation of methyl ketones (Haloform reaction) with X2/NaOH
- Hydrolysis of cyanides : Hydrolysis may be affected by acid or alkali
- Hydrolysis of an ester: with alkali or acid
- Hydrolysis of trihalogen derivative of alkanes
- Carboxylation of alkenes (Koch reaction)
- Reaction of Grignard Reagents with CO2
- By heating sodium alkoxide with CO
- From Sodium alkyl and CO2
- Catalytic oxidation of long chain hydrocarbons
- From alkynes
- By heating dicarboxylic acids eg oxalic acid or malonic acid
- By acidic hydrolysis of Malonic or aceto acetic ester
- By air oxidation of Acetaldehyde
- By air oxidation of Butane
- Quick vinegar process
GENERAL PROPERTIES
SOLUBILITY
MELTING POINTS
ACIDITY OF CARBOXYLIC ACIDS
CHEMICAL PROPERTIES
REDUCING CHARACTER OF FORMIC ACID
ACTION OF HEAT ON FORMATES
ACID DERIVATIVES
Ester
ACID CHLORIDES
- From acids
- From Salts (Industrial method)
- As acylating agent
- Determination of –NH2 and OH group in a molecule
ACID ANHYDRIDE (RCO)2O
(mixed anhydride)
ESTERS
AMIDES
UREA, CARBAMIDE (H2N.CO.NH2)
- Wohler synthesised in 1828, urea the first organic compound by heating ammonium cyanate
- By the action of ammonia on phosgene, ethyl carbonate, chloroformate or Urethanes.
- Manufacture
- It is used as fertilizer
- making barbiturates
- urea formaldehyde resin
- To improve octane number
- Stabilizer for explosives
SUBSTITUTED ACIDS
SATURATED DICARBOXYLIC ACIDS
- All are colourless crystalline solids, soluble in water. Solubility with increase in molecular weight, the odd acids are more soluble than even due to lower symmetry and poor packing.
- Their melting points follow the Oscillation or alternation rule which states that the melting point of an “even” acid is higher than that of the “odd” acid immediately below and above it in the series. It is also known as “saw-tooth” rule.
- Acid Strength: The strength of acids decreases from lower to higher member of the series as shown by the pka values
- Action of heat
TARTARIC ACID
- From Argol or Tartar - A brown coloured crystalline mass formed during the fermentation of grape juice known as argol is crystallised from hot water to get cream of tartar which contains impure (+) potassium hydrogen tartrate. Tartaric acid is obtained as follows :
- From Glyoxal
- From Fumaric acid
- From Maleic acid
- From dibromosuccinic acid
- It is used in silvering mirror
- Tartar emetic is used to cause nausea and vomiting in case of poisoning
- Pot. acid tartrate is used in Baking powder
- Rochelle salt is used in preparing Fehling solution.
CITRIC ACID
- From lemon juice
- From Sugar - Fermentation of molasses in presence of aspergillus niger or citromyces pfeferianus and inorganic salts. eg.: (NH4)2CO3, MgSO4 etc.
- From glycerol
It contain Copper Sulphate, Sodium Carbonate and Sodium Citrate. The structure of complex is
- Mg Citrate is used as laxative in medicine
- As mordant in dyeing and printing
- Ferric ammonium citrate as Iron tonic
- It is used in preparing acidulated soft drinks, jams, jellies, etc.
OXALIC ACID, ETHANEDIOIC ACID (COOH)2.2H2O
- Oxidation of Sucrose (Lab Method)
- Manufacture
- Hydrolysis of Cyanogen
- As a mordant in dyeing
- Volumetric analysis
- Removing ink stains
- In photography
BENZOIC ACID
PREPARATION
- By oxidation of alcohol or aldehyde
- By Oxidation of homologues of benzene : Oxidising agents dil. HNO3, alk. KMnO4, K2Cr2O7 + H2SO4
- By use of Grignard’s reagent
- Hydrolysis of Cyanides
- Manufacture of benzoic acid
SALICYCLIC ACID
- Kolbe-Schmidt reaction
- Reimer-Tiemann Reaction
