Free Chemistry NEET Notes for Hydrocarbons
Free Chemistry Notes available for Hydrocarbons (NEET)
Power up your NEET Exam prep with Chapter-Wise Chemistry Notes for Hydrocarbons at Onlineneetcoaching.in. Crafted by Brilliant Tutorial experts with 25 years of NEET insights.
- Hydrocarbons -
Important Chemistry Notes for IITJEE/NEET Preparation- Hydrocarbons
Class 11 Chemistry is a broad subject that requires a thorough understanding of the concepts and topics covered. As a result, we have provided Chemistry Notes PDF for IIT JEE/NEET to students and NEET aspirants. Hydrocarbons Class 11 Notes PDF for NEET can be found below. With the help of detailed syllabus, Class 11 students learn what they need to study, how many points are assigned to each unit, and how much time is allotted for each unit. As a result, they can easily plan their study schedule.
Check out the Hydrocarbons Class 11 notes PDF for your IIT JEE/NEET Preparation based on the IIT JEE/NEET Chemistry Syllabus. The Hydrocarbons notes PDF is designed in such a way that it is very useful for IIT JEE/NEET aspirants.
Hydrocarbons
SATURATED HYDROCARBONS, PARAFFINS OR ALKANES
They are open chain compounds of carbon and hydrogen having all the atoms linked together by single covalent bonds, least reactive in nature
(Parum = little; affinis = affinity) and having general formula CnH2n+2
NOMENCLATURE
TRIVIAL NAMES
The first four members have the trivial names derived from their preparation from corresponding alcohols containing same number of carbon atoms eg. methane from methyl alcohol, ethane from ethyl alcohol and so on. After butane they are named according to Latin or Greek numerals of the number of Carbon atoms present in them with class suffix-ane. eg. Pentane (penta = 5) : hexane (hexa = 6) and so on.
The straight chain hydrocarbons are called normal (n) and contain 1° or 1° & 2° carbon atoms. The branched chain hydrocarbons containing the group are called Iso and contain 1° & 3° or 1°, 2° and 3° carbon atoms. The hydrocarbons containing a quaternary carbon atom are called neo (new).
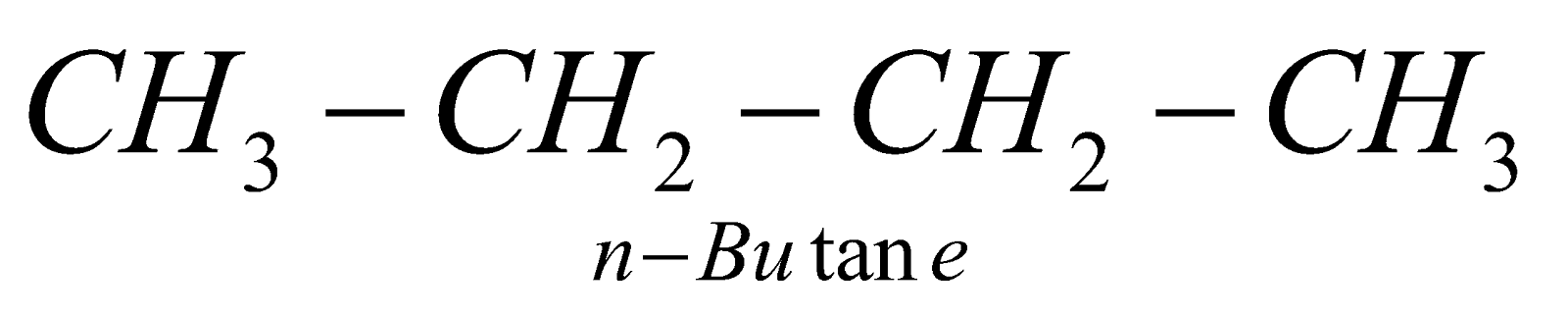 ;
; 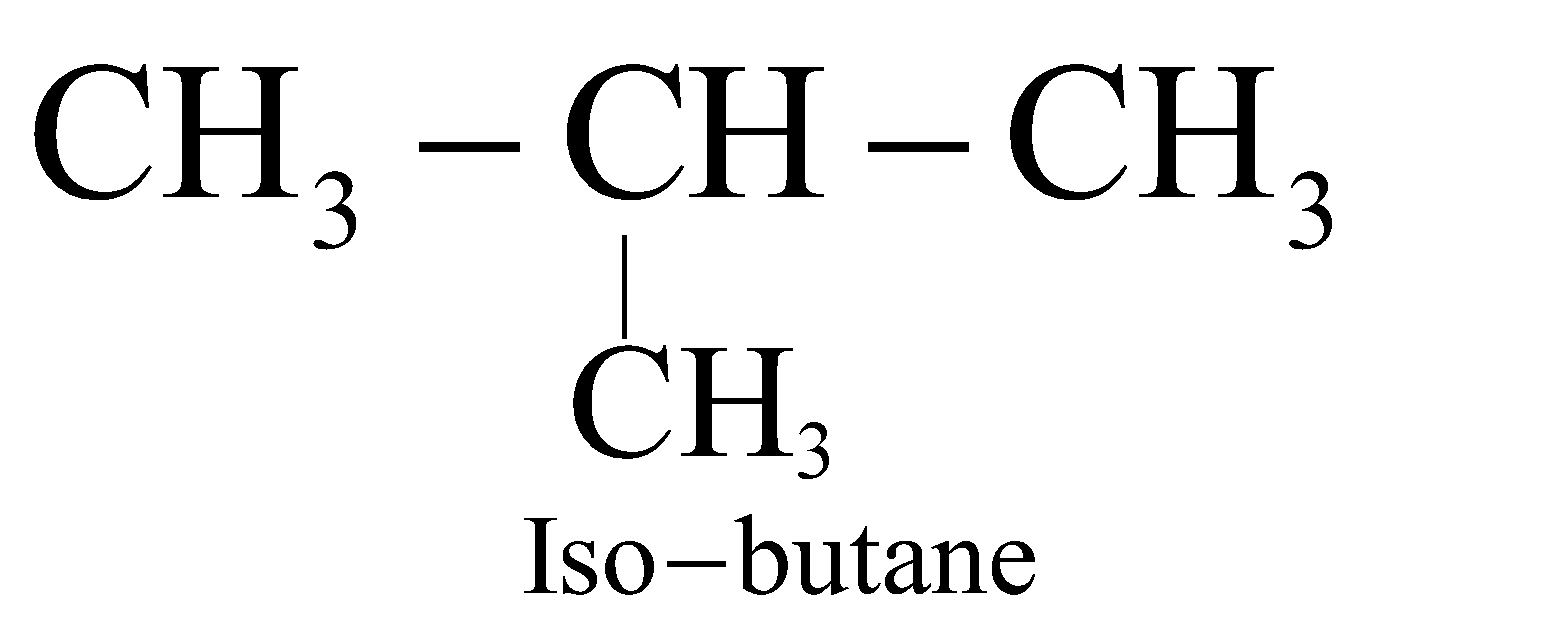 ;
; 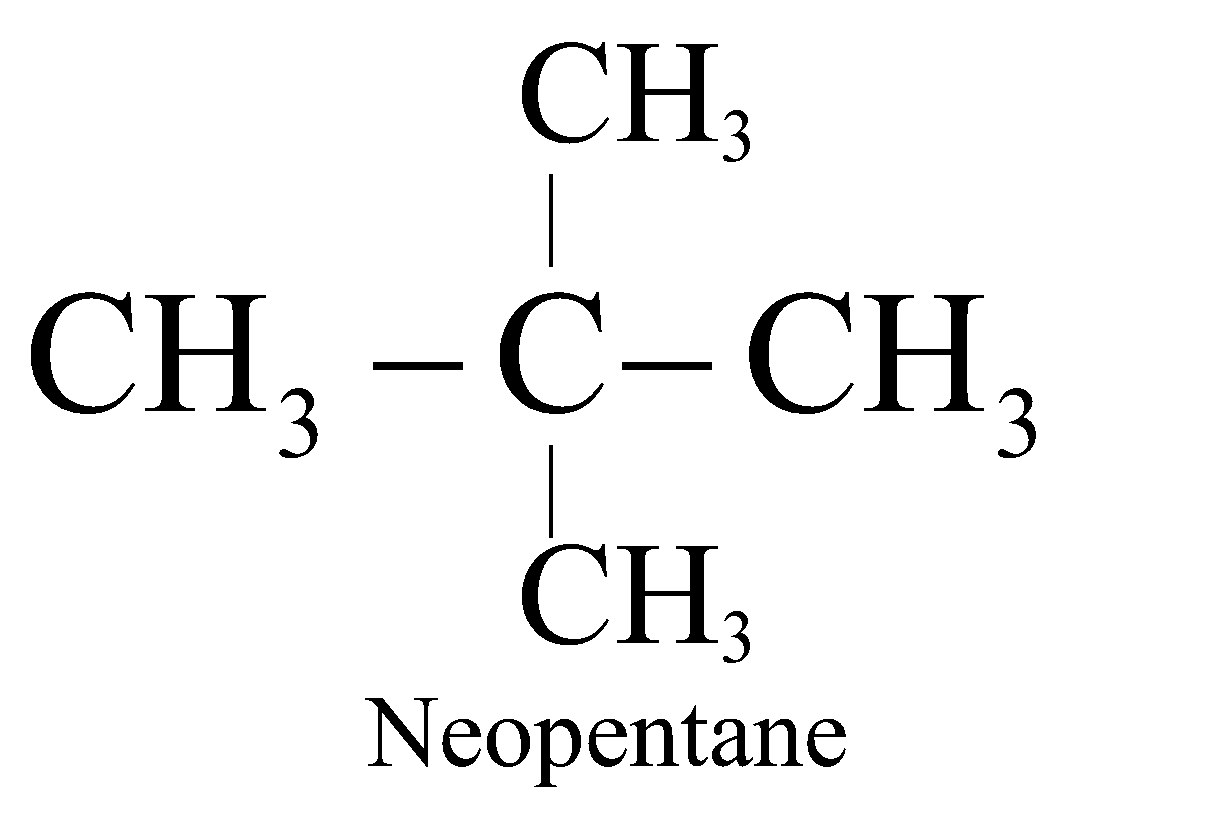
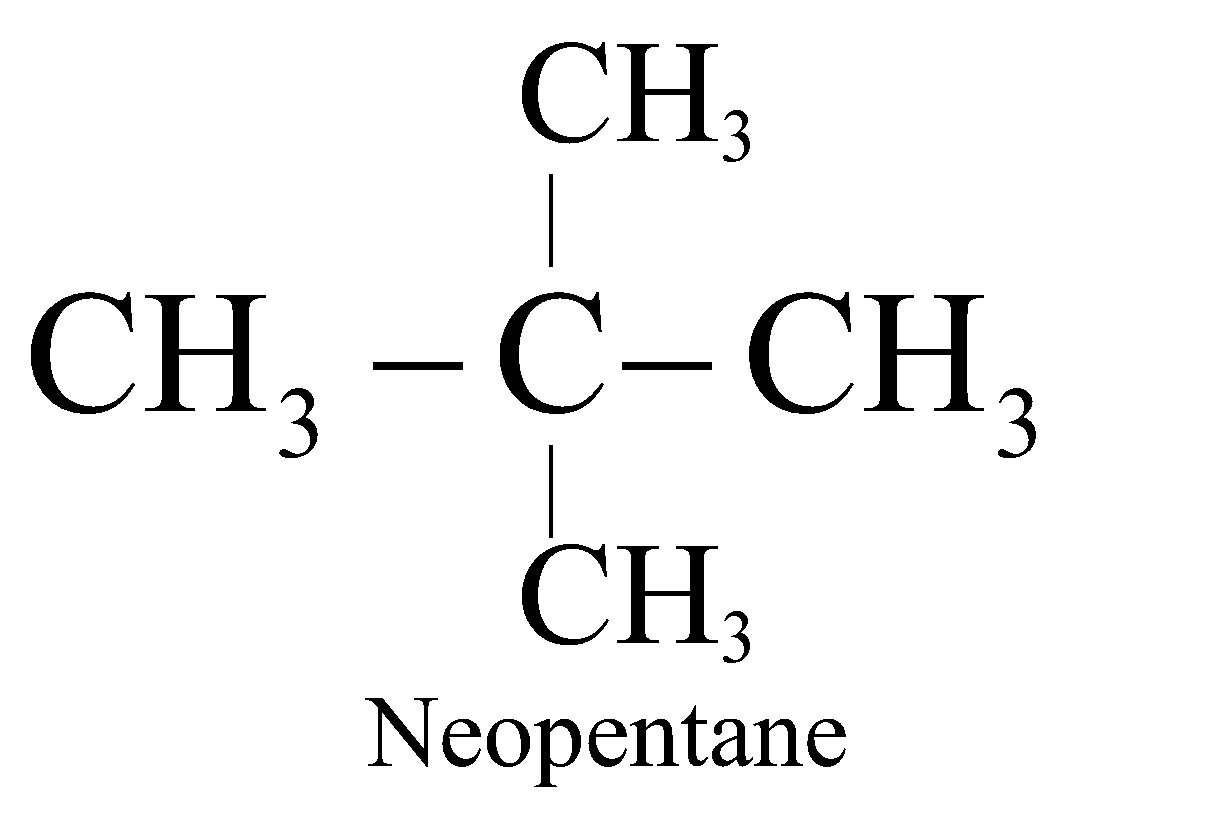 ;
;
DERIVED NAMES
In this system the higher hydrocarbons are considered as derived from methane, eg.
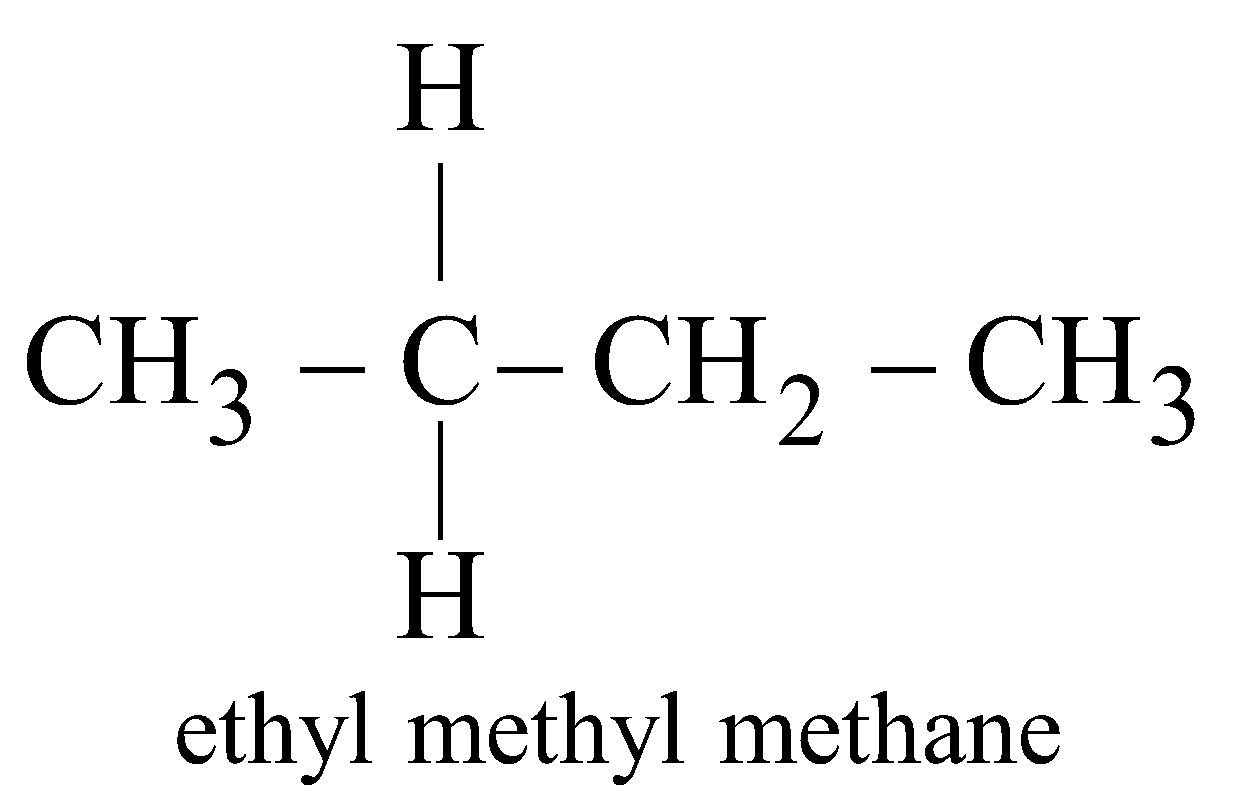 ;
;  ;
;
IUPAC SYSTEM
Types of carbon atoms :
Primary (1°) - It is attached to one carbon atom only.
Secondary (2°) - It is attached to two carbon atoms.
Tertiary (3°) - It is attached to three carbon atoms.
Quaternary (4°) - It is attached to four carbon atoms.
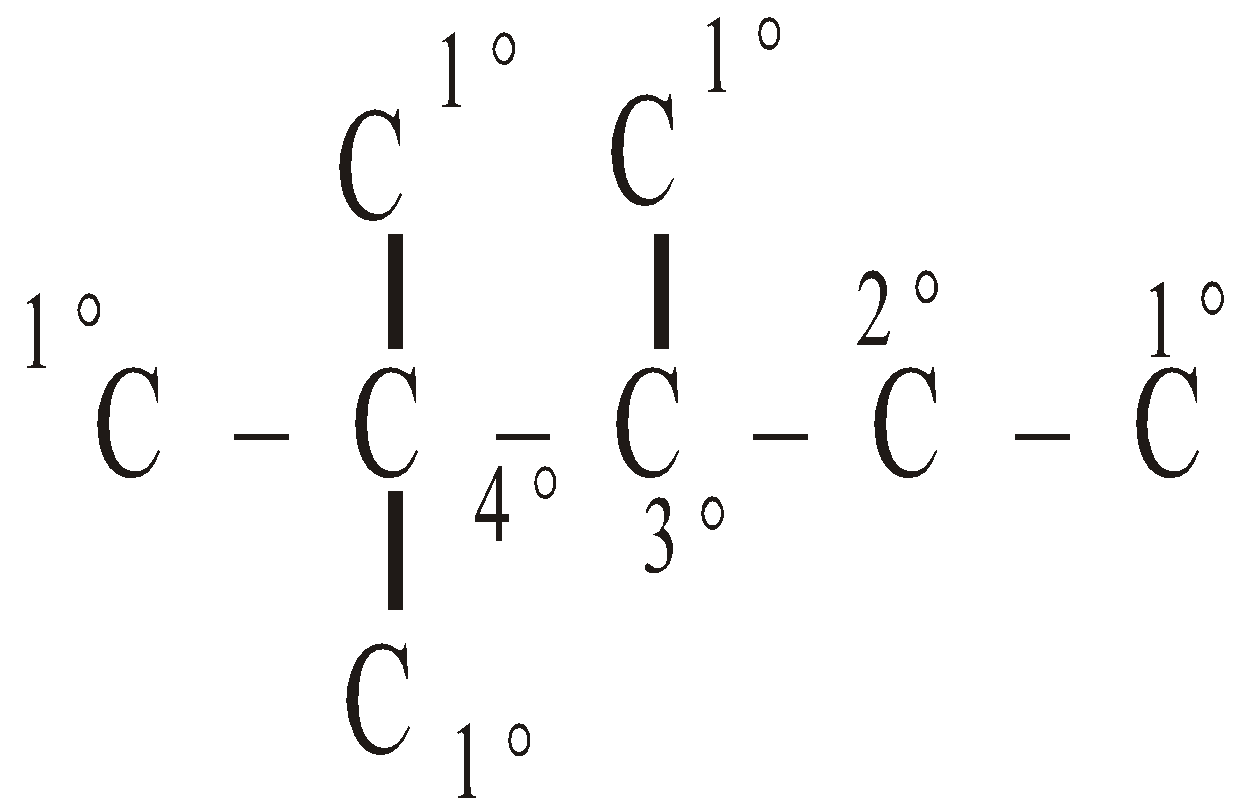
ISOMERISM
They exhibit chain isomerism
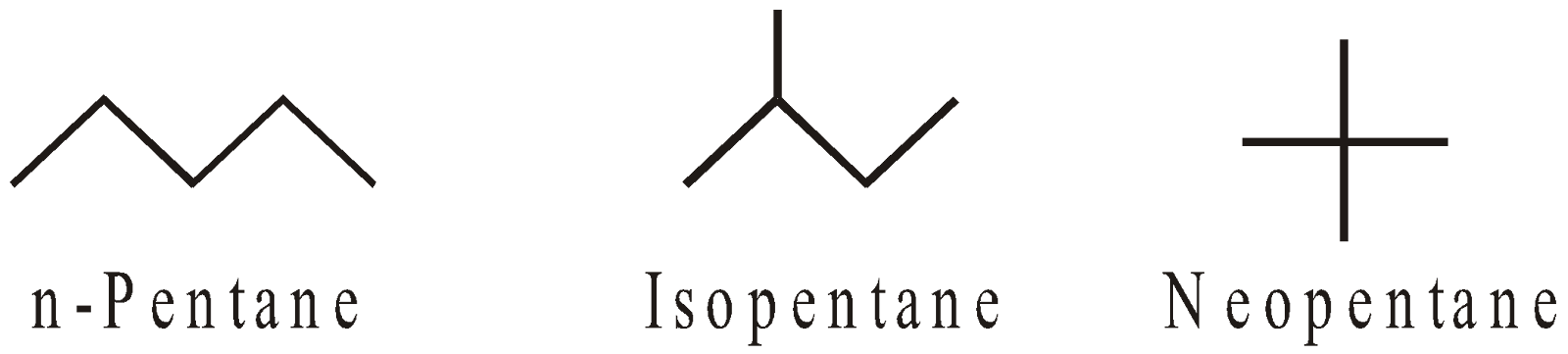 Number of isomers of alkanes :
Number of isomers of alkanes :

OCCURRENCE
The crude petroleum and natural gas contain hydrocarbons from C1 to C40. Ozokerite (a neutral wax) is a mixture of higher solid hydrocarbons. Waxes of some plants and animals also contain some higher paraffins
GENERAL METHODS OF PREPARATION OF ALKANES
-
From unsaturated hydrocarbons : (Sabatier and Senderens reaction)
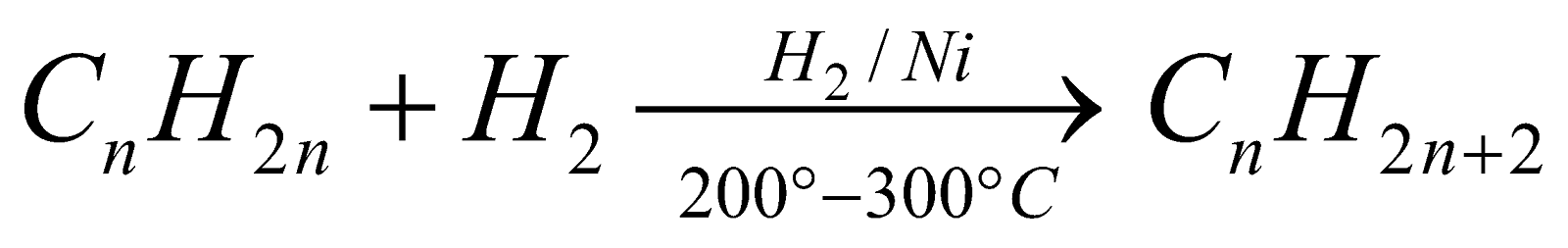
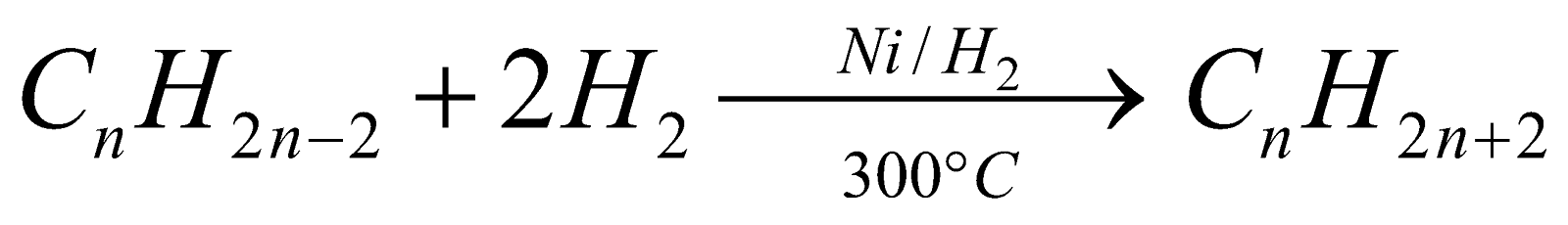
-
By heating anhydrous sodium salt of fatty acid with soda lime (Lab method).

-
By reduction of alkyl halides with nascent hydrogen.
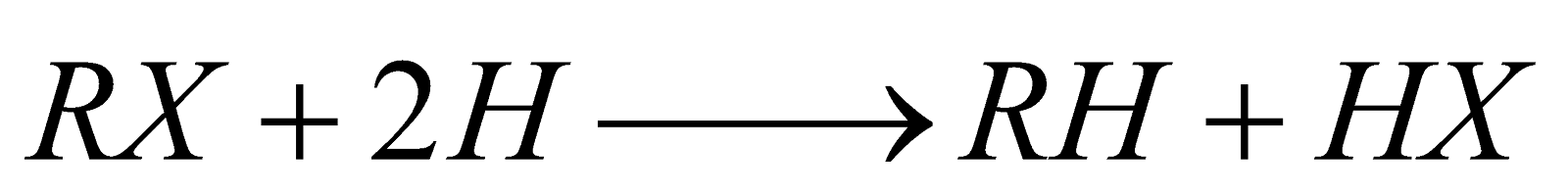 Reducing agents used are Na/C2H5OH; Zn/HCl;
Zn–Cu couple; Hg–Al couple, HI/P.
Reducing agents used are Na/C2H5OH; Zn/HCl;
Zn–Cu couple; Hg–Al couple, HI/P.
-
Wurtz synthesis :
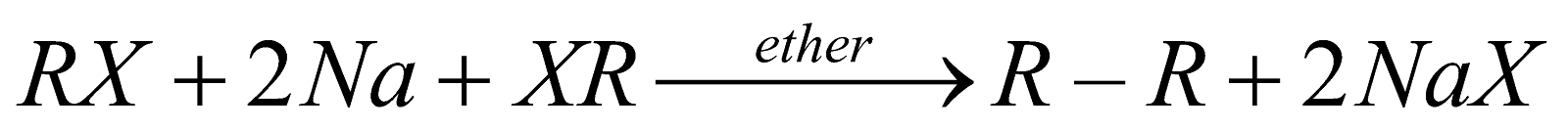 Alkanes with odd number of carbon atoms can not be prepared by this method.
Different alkyl halides give mixture of hydrocarbons viz. R – R, R' – R' & R – R'.
3° alkyl halides give alkenes by dehydrohalogenation.
Alkanes with odd number of carbon atoms can not be prepared by this method.
Different alkyl halides give mixture of hydrocarbons viz. R – R, R' – R' & R – R'.
3° alkyl halides give alkenes by dehydrohalogenation.
-
By Frankland's method :
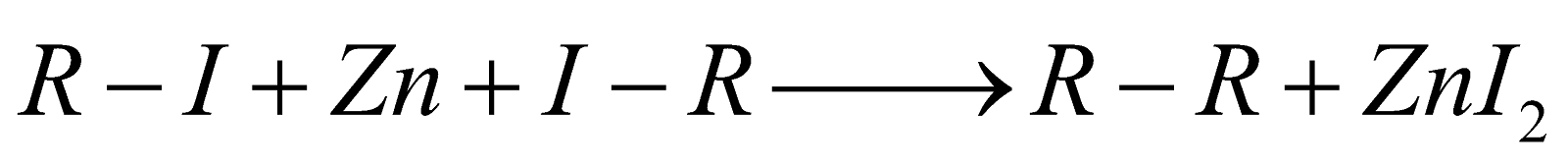
-
From Grignard's reagent :
 H must be attached to O, N, S or sp hybridised C-atom. (i.e. active H-atom).
H must be attached to O, N, S or sp hybridised C-atom. (i.e. active H-atom).
-
Kolbe's electrolytic method :

-
By Clemmensen reduction : From carbonyl compounds

-
By Wolf. Kishner reduction : From Carbonyl compounds

-
By Berthelot's reaction :

-
From metal carbides :

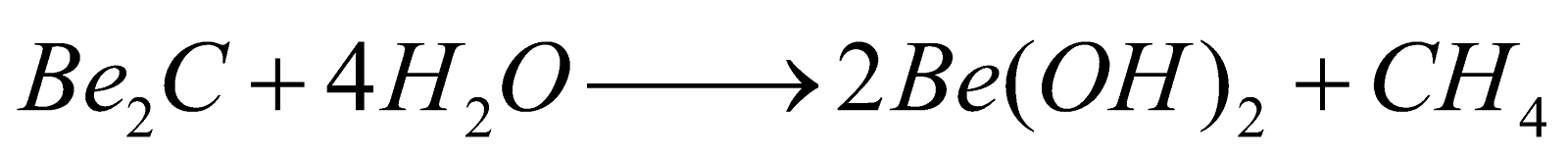
-
From carbon monoxide
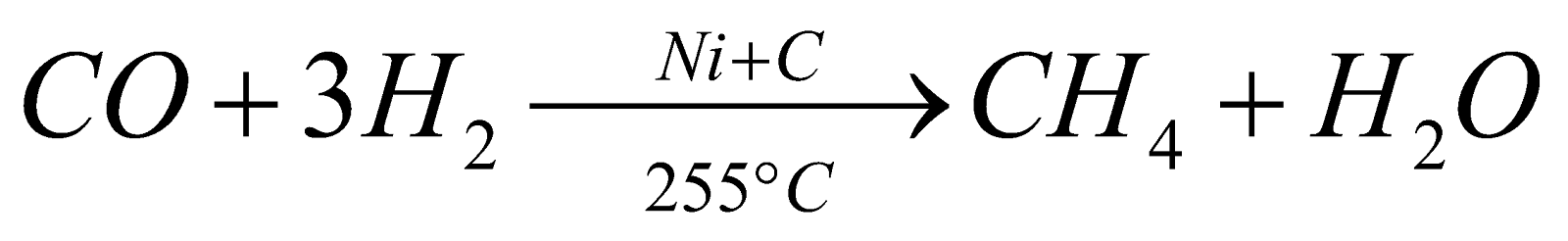
-
By reduction of alcohols, aldehydes, ketones and carboxylic acids :
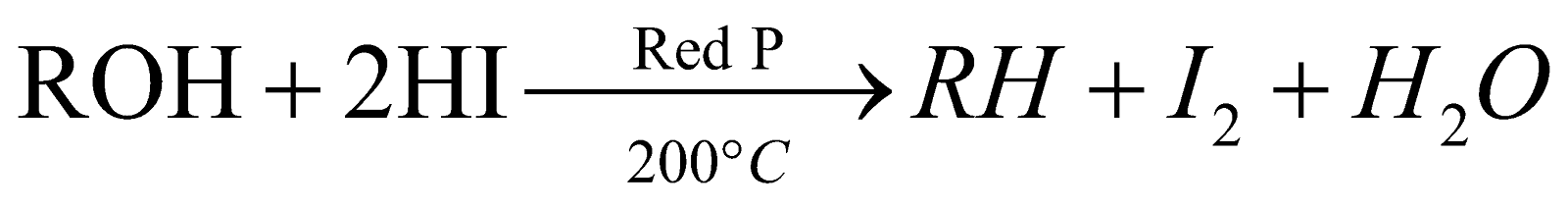

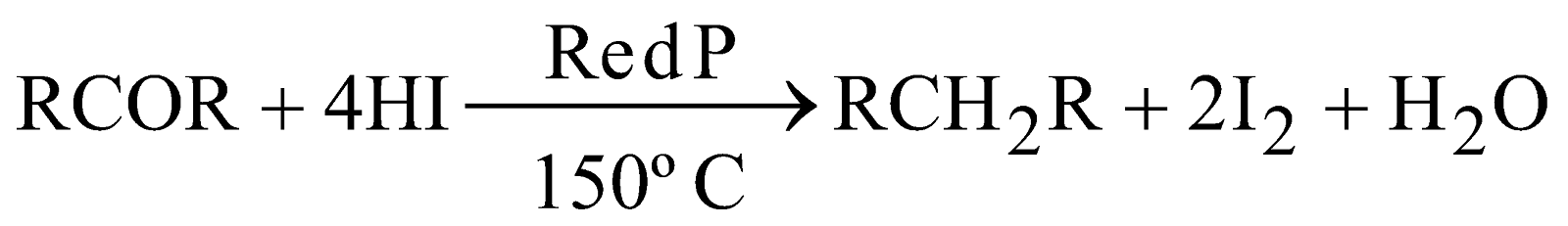
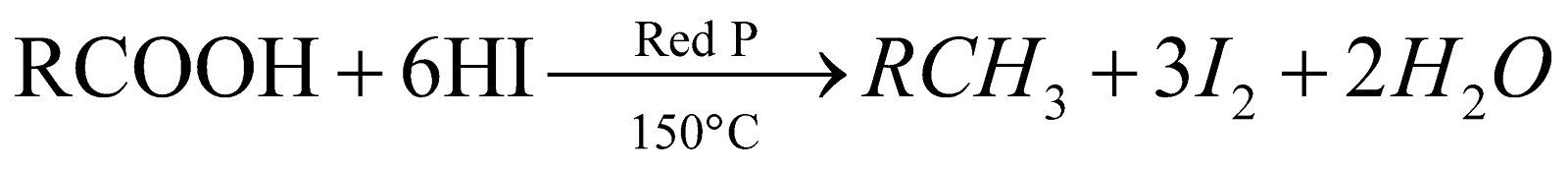
-
Corey-House alkane synthesis :

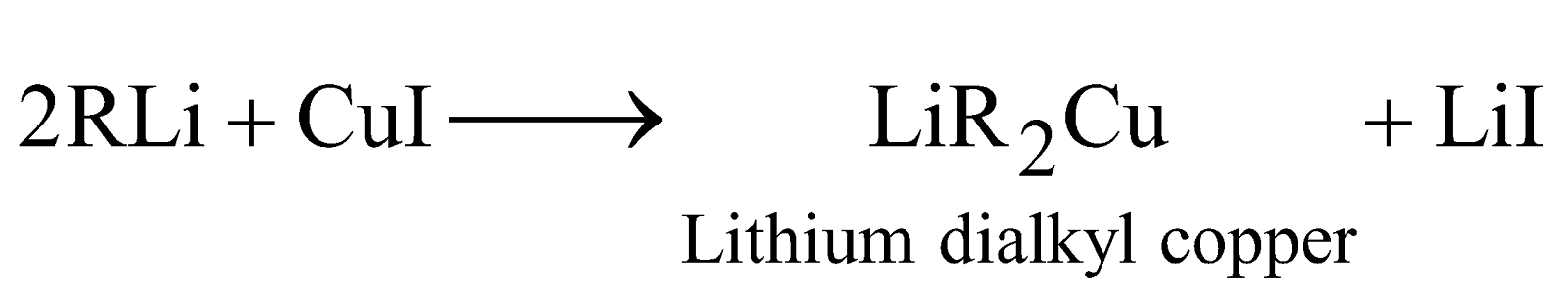
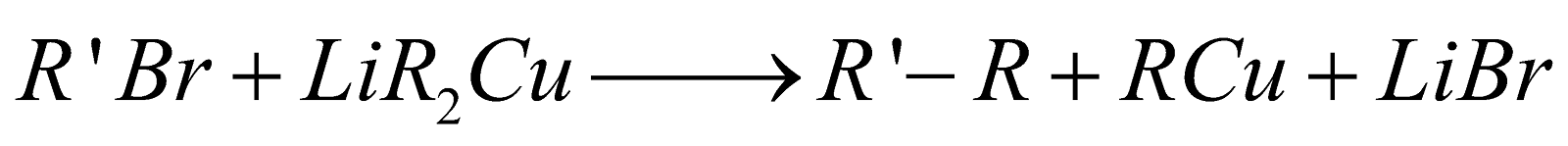 The reaction is particularly useful for preparing unsymmetrical alkanes.
The reaction is particularly useful for preparing unsymmetrical alkanes.
PROPERTIES
C1–C4 gases; C5–C17 colourless liquids, higher solids. Insoluble in water soluble in organic solvents
Branched chain alkanes boil at lower temperature than isomeric straight chain alkanes. The latter have higher van der Waals forces of attraction.
Melting points of hydrocarbons containing even number of C-atoms are relatively more than those containing odd number of carbon atoms.

CONFORMATIONAL ANALYSIS
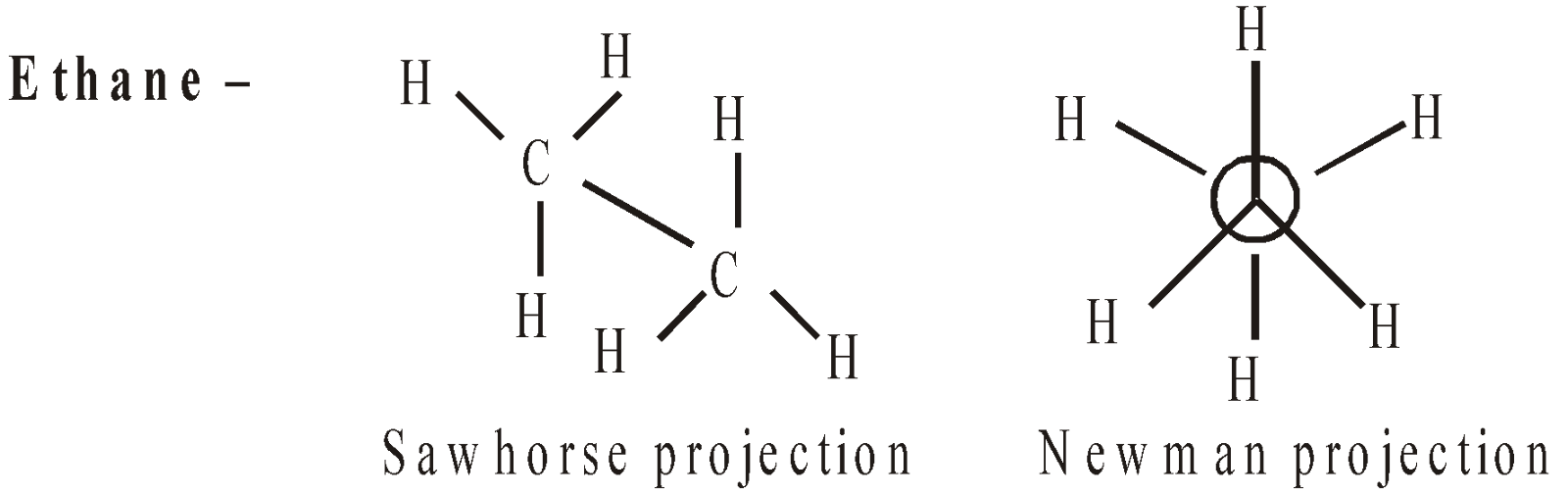 Different arrangements of atoms in a molecule convertible into one another by rotation about single bond are called conformers or conformations. Their study is known as conformational analysis. If the energy barrier to the rotation is nil or small, the rotation is said to be free or almost free. Ethane can exist in an infinite number of conformations.
Different arrangements of atoms in a molecule convertible into one another by rotation about single bond are called conformers or conformations. Their study is known as conformational analysis. If the energy barrier to the rotation is nil or small, the rotation is said to be free or almost free. Ethane can exist in an infinite number of conformations.
 Ө = It is dihedral angle between C–H bonds on the front of a Newman projection and those on the back.
Order of stability : Staggered > Skew > Eclipsed
Energy barrier between eclipsed and staggered is 2.8 kcal/mol.
Ө = It is dihedral angle between C–H bonds on the front of a Newman projection and those on the back.
Order of stability : Staggered > Skew > Eclipsed
Energy barrier between eclipsed and staggered is 2.8 kcal/mol.
Conformations of Propane C3H8 :
 Order of stability : Staggered > Skew > Eclipsed
Energy barrier between eclipsed and staggered is 3.3 kcal/mol
Order of stability : Staggered > Skew > Eclipsed
Energy barrier between eclipsed and staggered is 3.3 kcal/mol
Conformations of n-Butane C4H10 :
 Order of stability : Anti > Gauche > Skew > Eclipsed > Fully Eclipsed
Energy barrier between fully eclipsed and fully staggered (anti) is 5.3 kcal / mol or 22 kJ/mol.
Order of stability : Anti > Gauche > Skew > Eclipsed > Fully Eclipsed
Energy barrier between fully eclipsed and fully staggered (anti) is 5.3 kcal / mol or 22 kJ/mol.
Conformations of Cyclohexane :
It exists in two nonplanar, strainless forms, the boat and the chair form.
 Equatorial hydrogens lie in the plane of the ring carbons.
Axial hydrogens lie (up or down) the plane of the ring.
There are six equatorial and six axial hydrogens. In the flipping and reflipping between conformations, axial becomes equatorial and vice versa. Chair form has the lowest energy.
Equatorial hydrogens lie in the plane of the ring carbons.
Axial hydrogens lie (up or down) the plane of the ring.
There are six equatorial and six axial hydrogens. In the flipping and reflipping between conformations, axial becomes equatorial and vice versa. Chair form has the lowest energy.
Cyclohexane can assume other shapes also.
 BAEYER'S STRAIN THEORY
When ring compounds are formed, the bonds deviate from normal positions which produces a condition of strain in the molecule. The strain is directly proportional to angle of deviation and can be calculated as follows. The more the angle of deviation, the less is the stability.
Angle of deviation of cyclic compound =
BAEYER'S STRAIN THEORY
When ring compounds are formed, the bonds deviate from normal positions which produces a condition of strain in the molecule. The strain is directly proportional to angle of deviation and can be calculated as follows. The more the angle of deviation, the less is the stability.
Angle of deviation of cyclic compound = 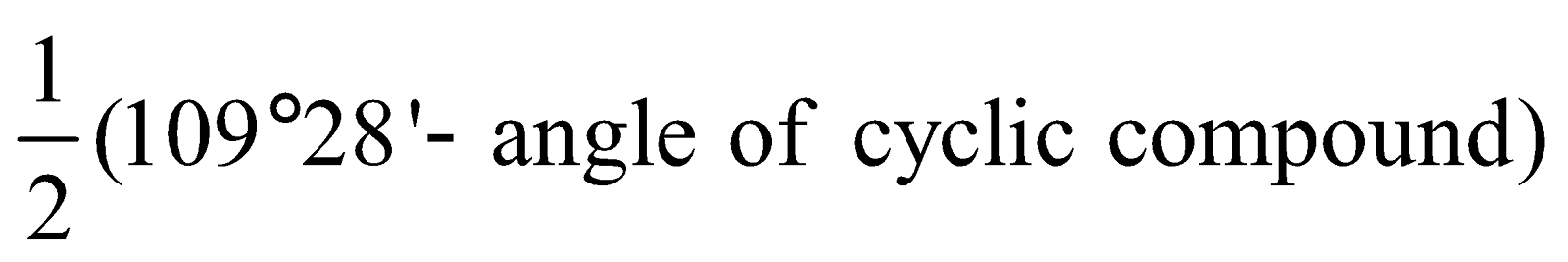 For Cyclopropane =
For Cyclopropane =  Cyclobutane =
Cyclobutane = 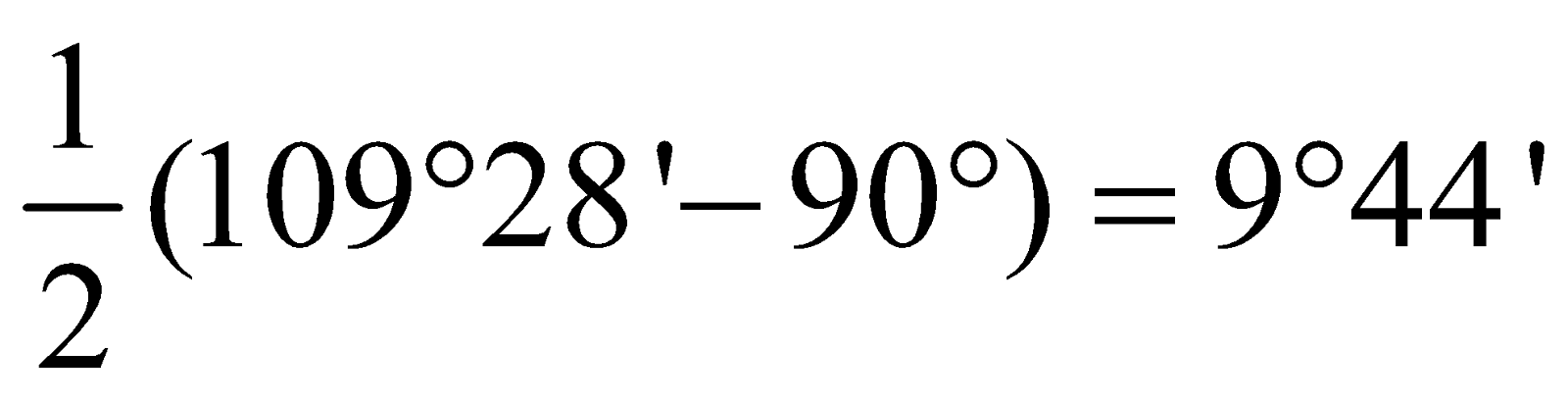 Cyclopentane =
Cyclopentane = 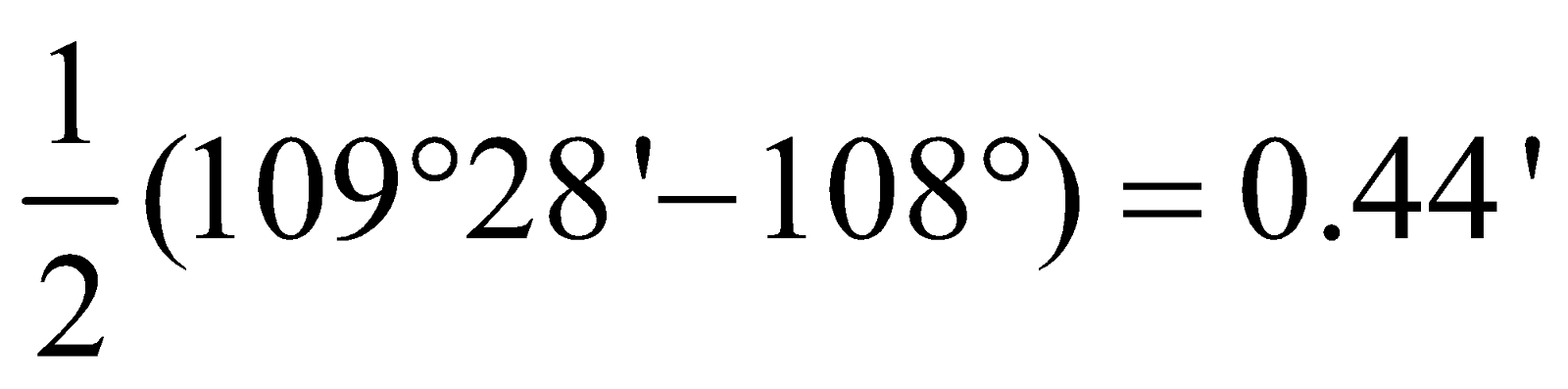 Cyclohexane =
Cyclohexane = 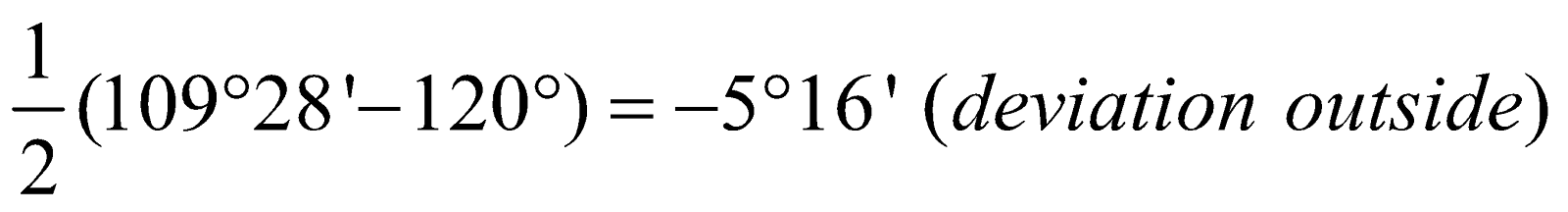 Cycloheptane =
Cycloheptane = 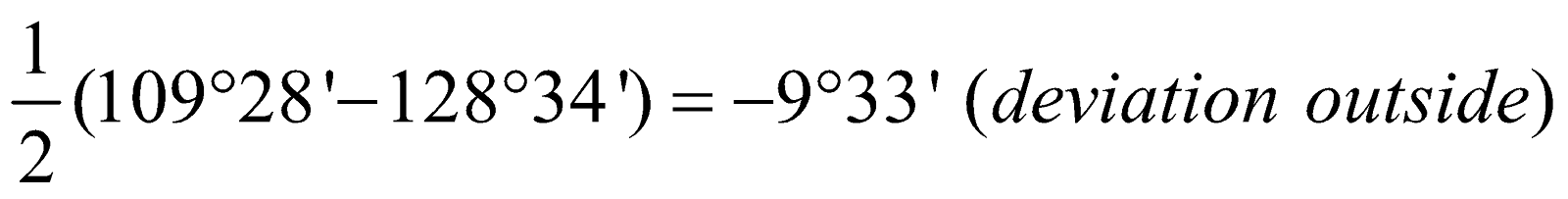 Limitations : Baeyer's strain theory fails to explain the stability of large ring alicyclic compounds.
Limitations : Baeyer's strain theory fails to explain the stability of large ring alicyclic compounds.
SACHSE MOHR THEORY OF STRAINLESS RINGS
A ring with six or more carbon atoms can assume "puckered" structure and there is a little distortion of normal tetrahedral angle. Thus there is little or negligible angle strain in the molecule.
UNSATURATED HYDROCARBONS, OLEFINS OR ALKENES
They are open chain compounds of carbon and hydrogen having double bonds also known as Olefins (oil forming) Olefiant gas is Dutch name of ethylene which formed oily ethylene chloride with chlorine. Their general formula is CnH2n.
NOMENCLATURE
COMMON SYSTEM : The suffix -ane of alkane is replaced by -ylene, and named as Alkylenes eg. ethylene, propylene etc.
IUPAC SYSTEM : The suffix -ane of alkane is replaced by -ene and hence named as Alkenes.
DERIVED NAMES : They are named as substituted derivatives of ethylene.
ISOMERISM
Alkenes show four types of isomerism
-
Chain Isomerism

-
Position Isomerism

-
Ring Chain Isomerism
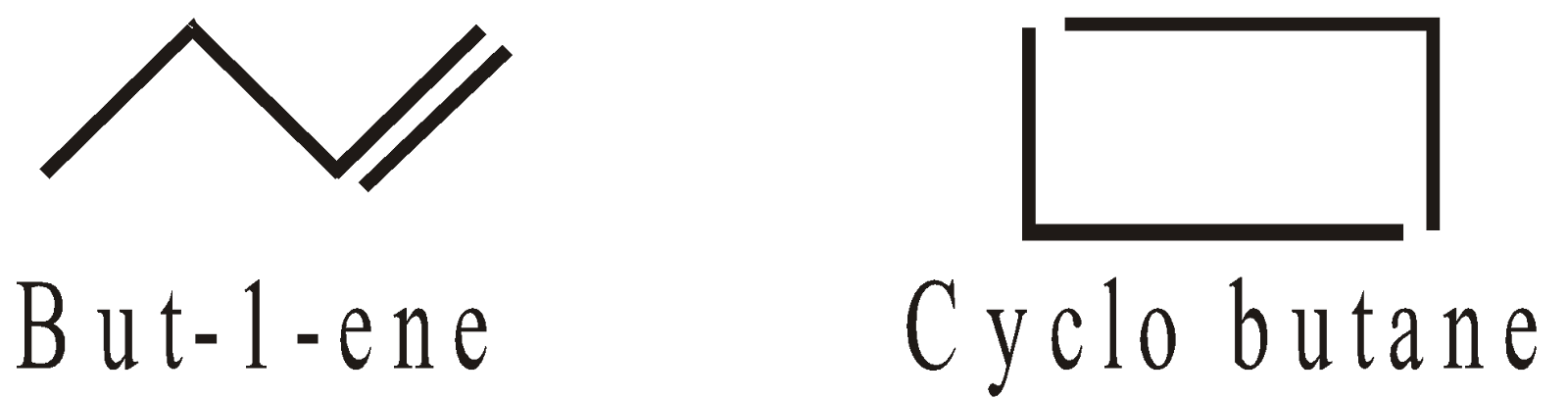
-
Geometrical Isomerism

GENERAL METHODS OF PREPARATIONS OF ALKENES
-
By dehydration of alcohols :
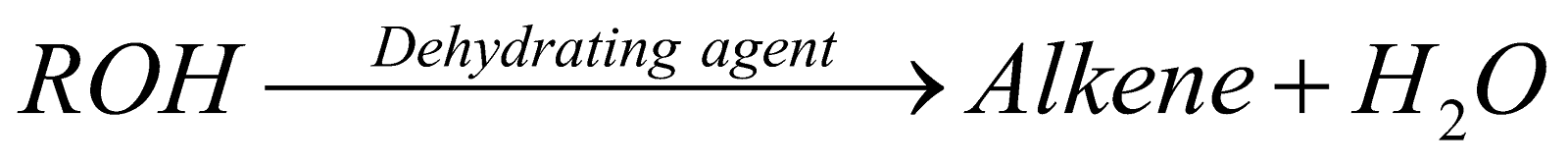
Dehydrating agents Conc. H2SO4; P2O5; H3PO4; Al2O3. Anhy. ZnCl2. Anhy. Oxalic acid.
Ease of dehydration of alcohols 3° > 2° > 1°.
-
By Dehydrohalogenation of Alkyl halides.

-
Dehydrohalogenation and dehydration follows Saytzeff's rule (Hydrogen is removed from C-atom containing lesser number of H-atoms or more substituted alkene is formed)
-
More substituted alkenes are more stable.
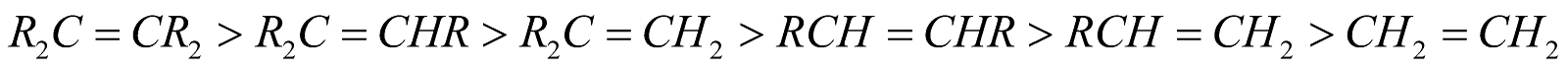
-
Ease of dehydrohalogenation 3° > 2° > 1°.
-
Ease of dehydrohalogenation : Iodides > Bromides > Chlorides
-
By dehalogenation of vicinal halides :

-
By dehalogenation of gem. halides :

-
By electrolysis of Sodium or potassium salt of succinic acid or its derivatives
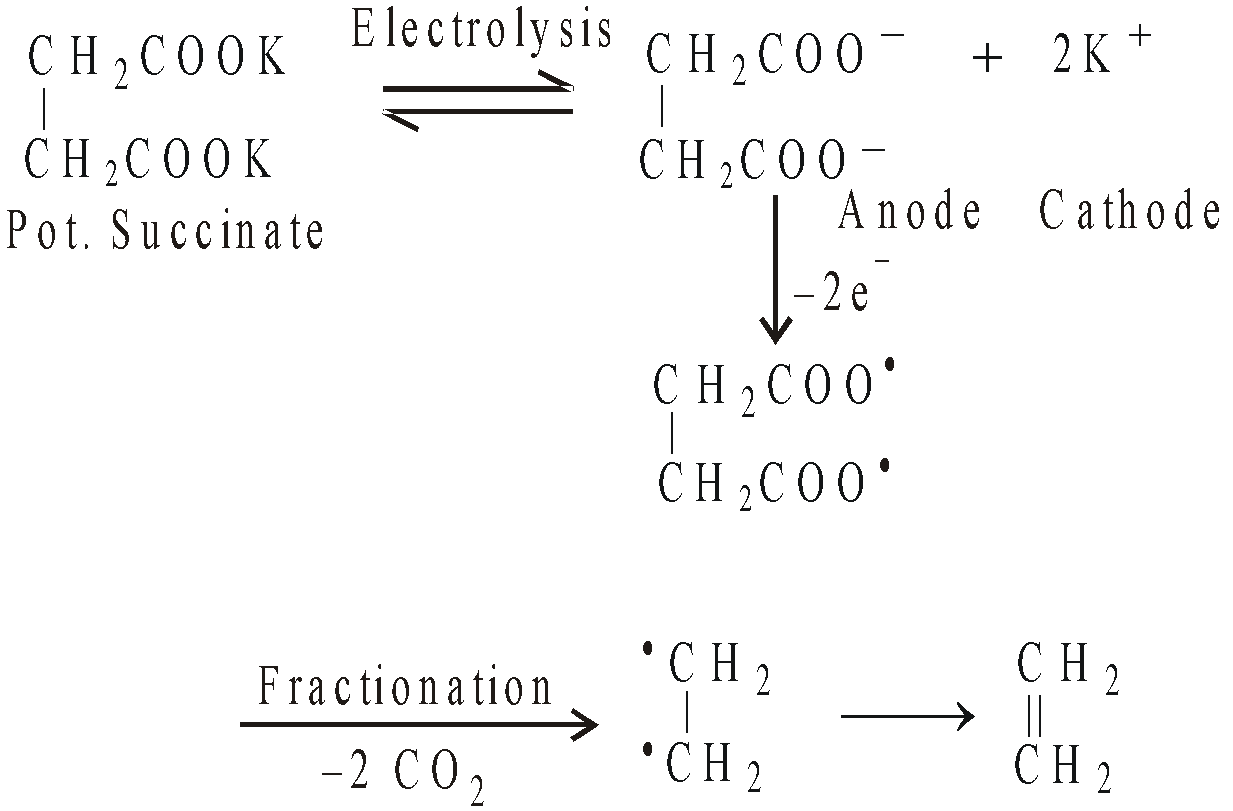 Alkenes with odd or even number of carbon atoms and having any position of double bond can be prepared.
Alkenes with odd or even number of carbon atoms and having any position of double bond can be prepared.
-
By partial reduction of alkynes :
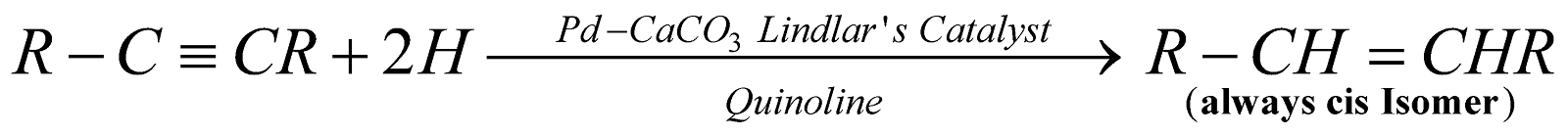

-
Decomposition of quaternary ammonium hydroxide:

-
By cracking of alkanes :

PROPERTIES
C2–C4 gases; C4 – C15 liquids C16 onwards Solids. Less volatile than alkanes and possess anaesthetic properties.
CHEMICAL PROPERTIES
Addition reactions given by alkenes are known as electrophilic addition reactions.





ALKYNES OR ACETYLENES
They are characterised by the presence of triple bond having general formula CnH2n–2.
NOMENCLATURE
According to trivial system they are regarded as derivatives of acetylene. In the IUPAC system their names are derived by replacing suffix -ane by -yne.
Formula
Common Name
Derived name
IUPAC name
CH ☰ CH
Acetylene
Acetylene
Ethyne
CH3C ☰ CH
Allylene
Methyl acetylene
Propyne
CH3C ☰ C– CH3
Cretonylene
Dimethyl acetylene
But-2-yne
ISOMERISM
They exhibit four types of Isomerism
-
Chain Isomerism

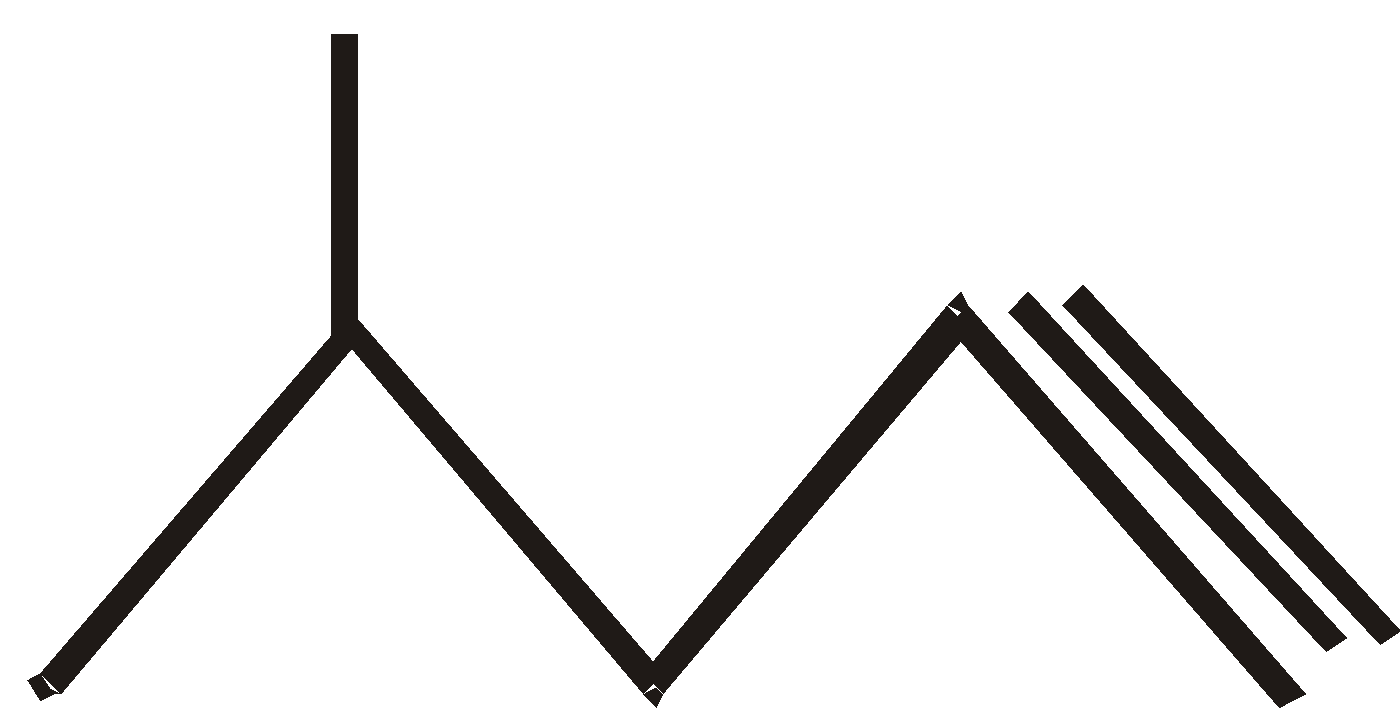 Hex–1–yne 4–methyl pent –1– yne
Hex–1–yne 4–methyl pent –1– yne
-
Position Isomerism

 Hex – 1–yne Hex – 3 – Yne
Hex – 1–yne Hex – 3 – Yne
-
Functional Isomerism
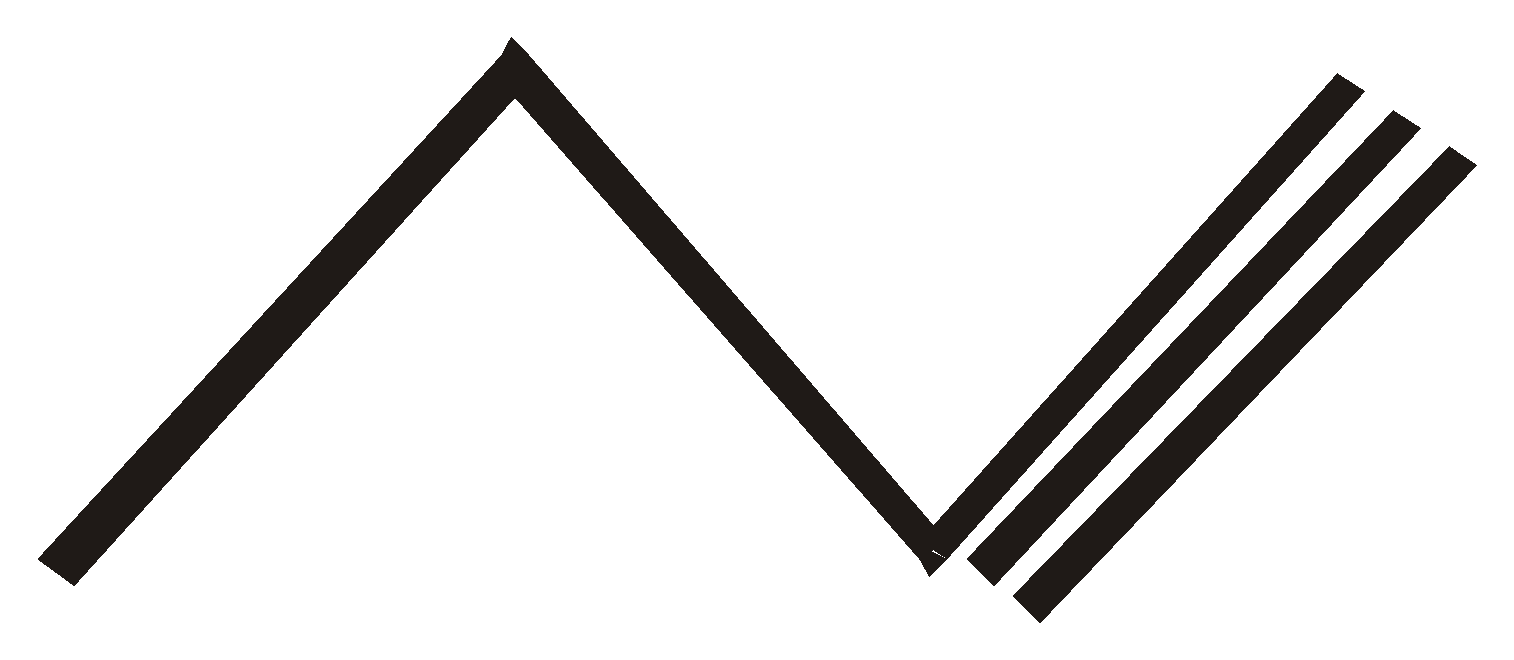
 But – 1 –yne But –1,3 – diene
But – 1 –yne But –1,3 – diene
-
Ring chain Isomerism

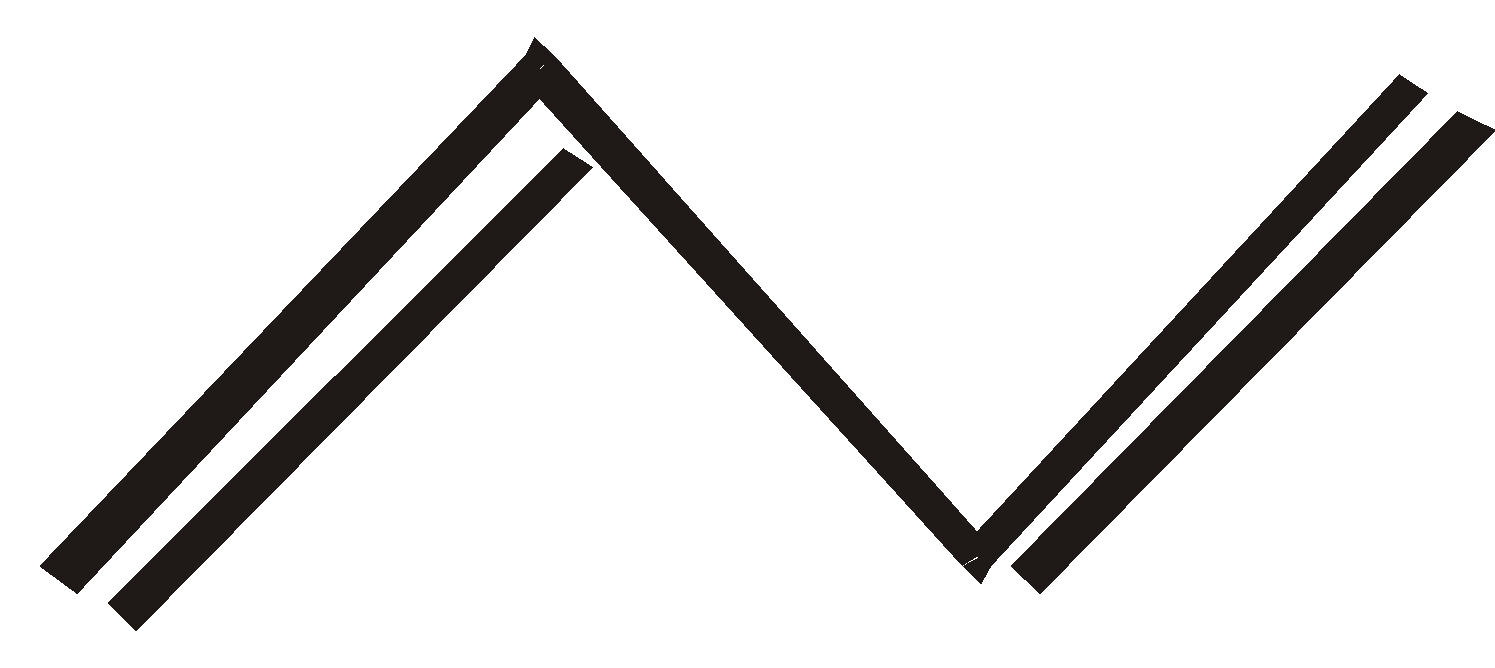
 But – 2 –yne cyclobutene
But – 2 –yne cyclobutene
GENERAL METHODS OF PREPARATION
-
By dehydrohalogenation of vicinal halides
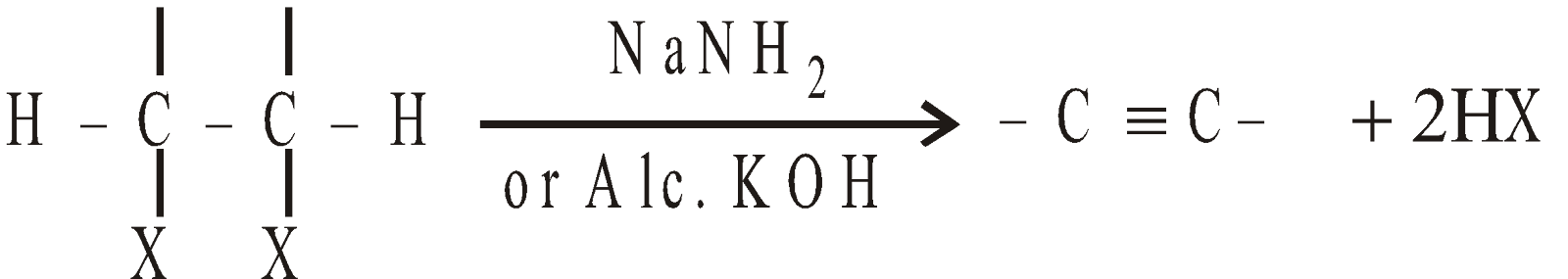
-
By dehydrohalogenation of gem halides
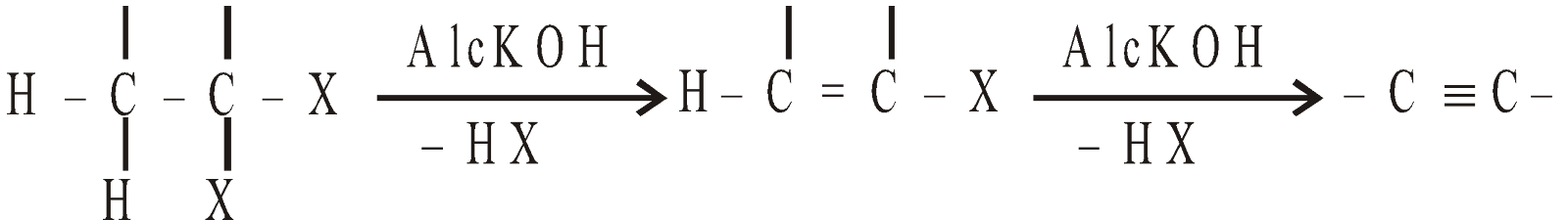
-
By dehalogenation of tetrahalides
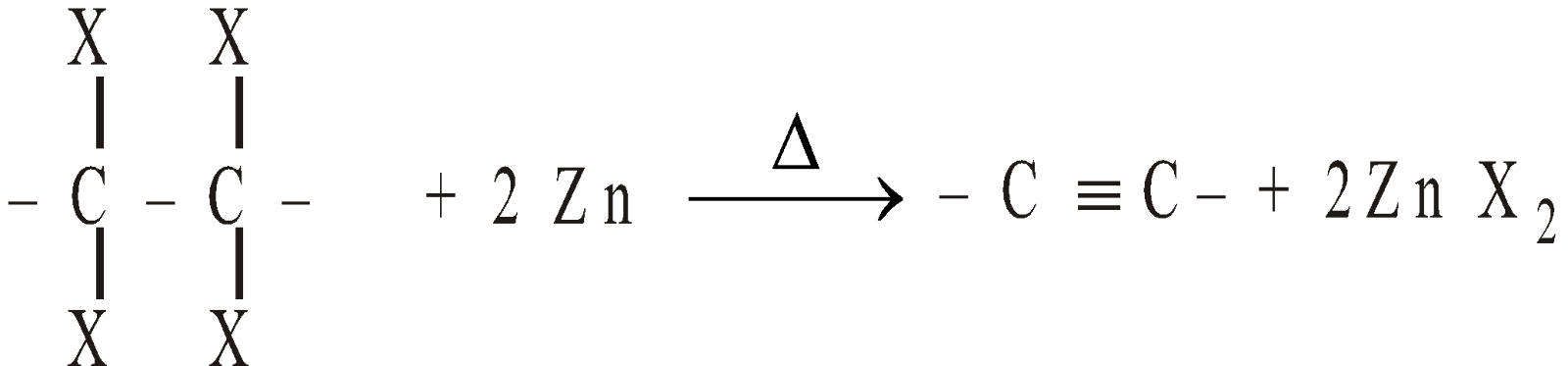
-
From lower alkynes
HC  CH
CH  NaC
NaC  CNa
CNa 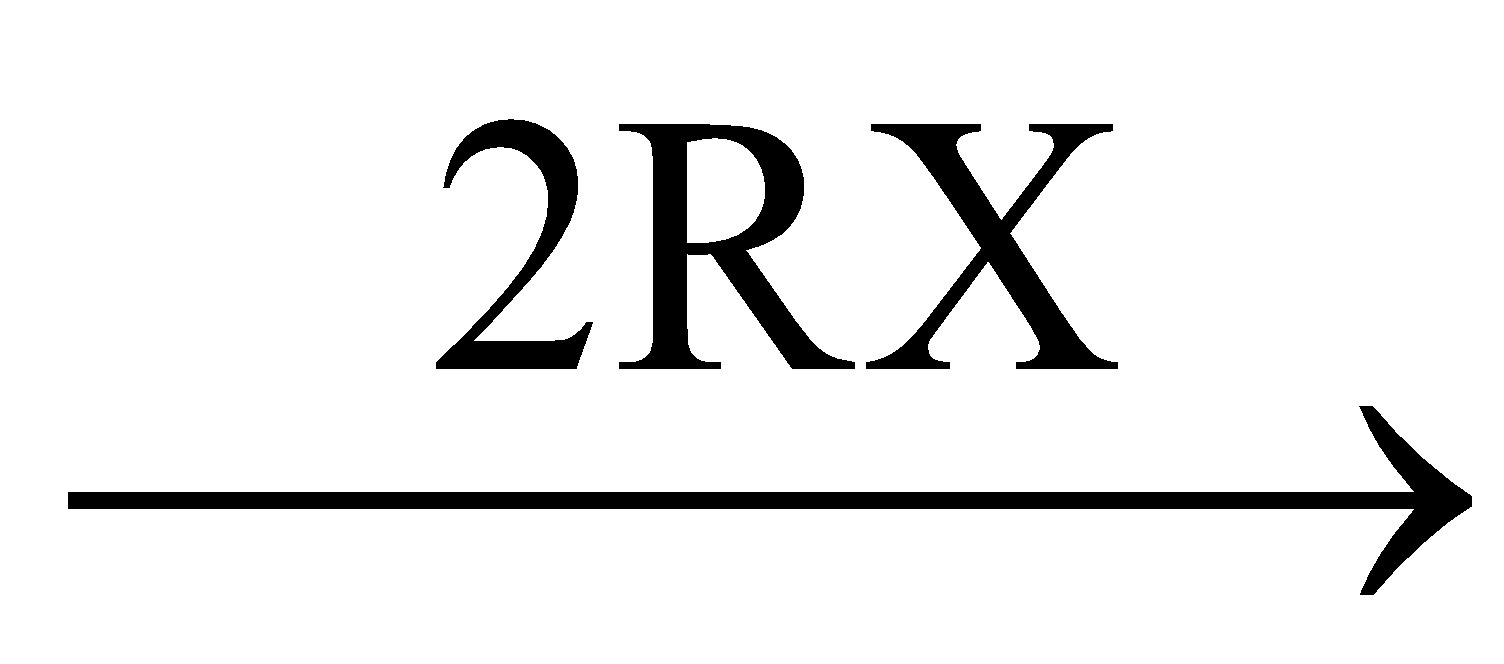 R – C
R – C  C – R + 2NaX
C – R + 2NaX
-
Kolbe's electrolytic method

-
From haloform
CHI3 + 6Ag + I3CH  CH
CH  CH + 6AgI
CH + 6AgI
-
Acetylene from calcium carbide (wohler's reaction)
CaC2 +2H2O  Ca(OH)2 + CH
Ca(OH)2 + CH  CH
CH
-
Berthlot's reaction
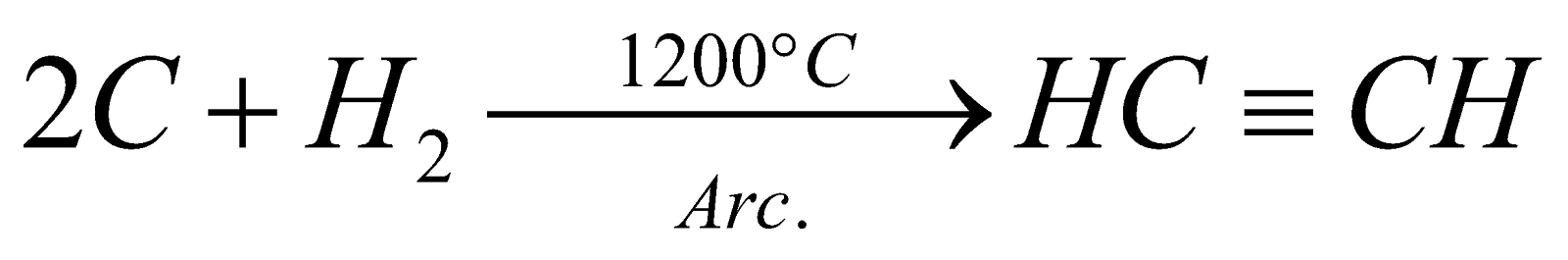
-
From methane :

PROPERTIES
C2–C4 gases C5–C12 liquids. C13 onwards solids. Acetylene has garlic odour due to phosphene and hydrogen sulphide impurity.
CHEMICAL PROPERTIES
Alkynes are less reactive than alkenes for electrophilic addition reactions.

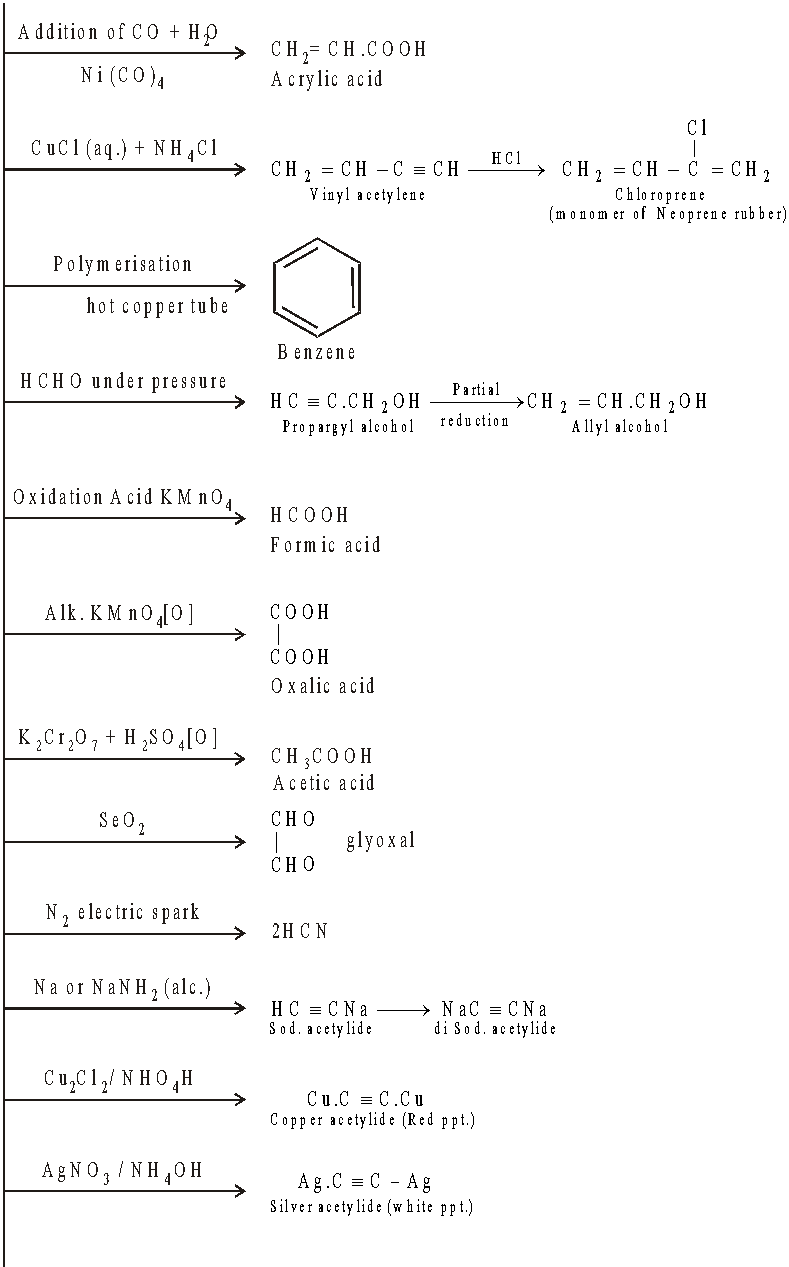
AROMATIC HYDROCARBONS, BENZENE C6H6
PREPARATION
SMALL SCALE PREPARATION
-
From acetylene :
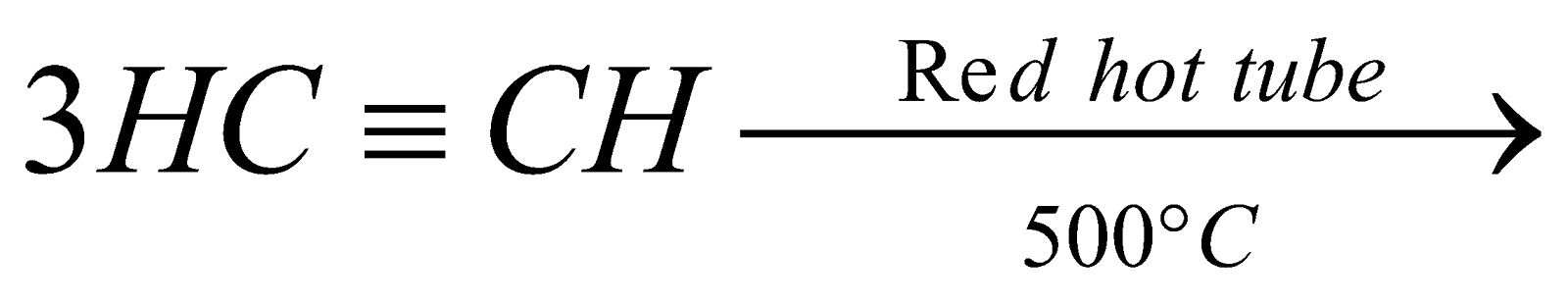

-
From benzoic acid :

-
From Phenol :

-
From Chlorobenzene :
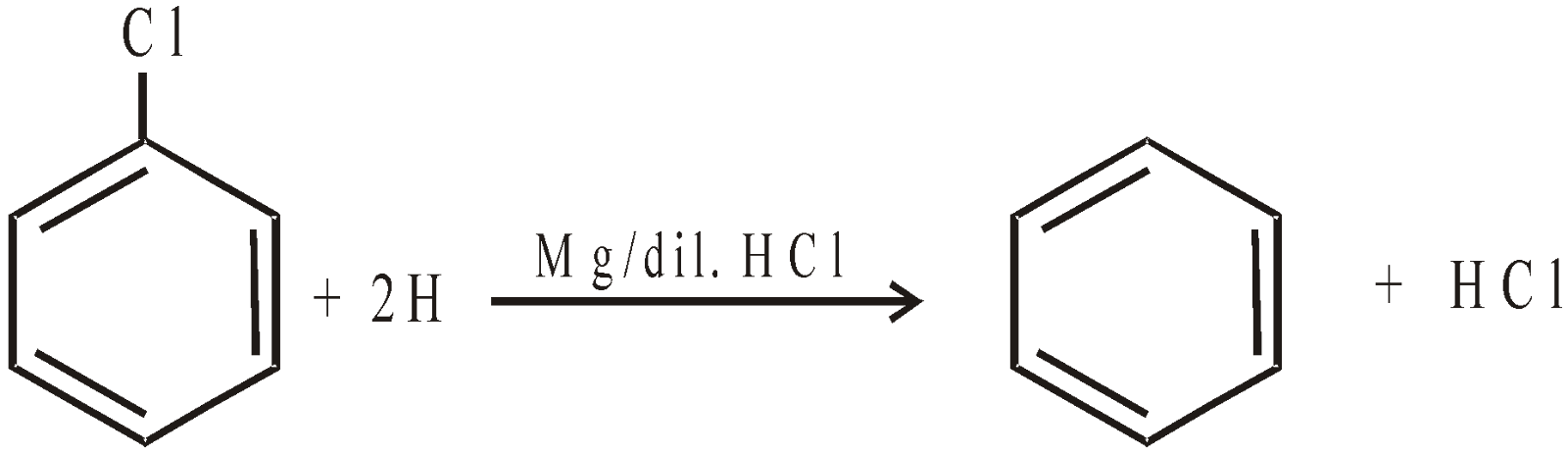
-
From benzene sulphonic acid :
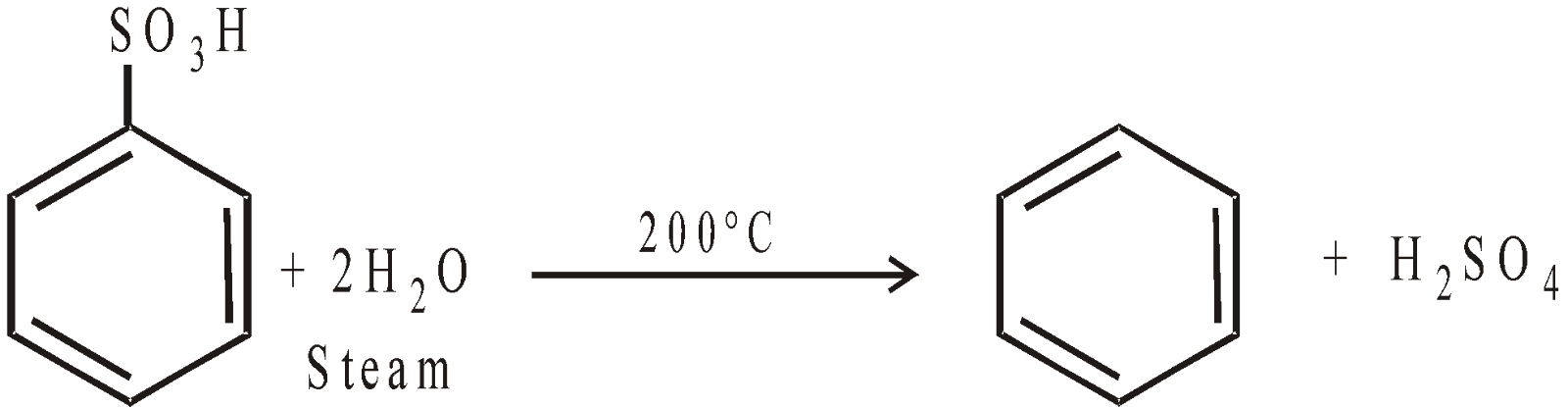
-
From benzene diazonium chloride :
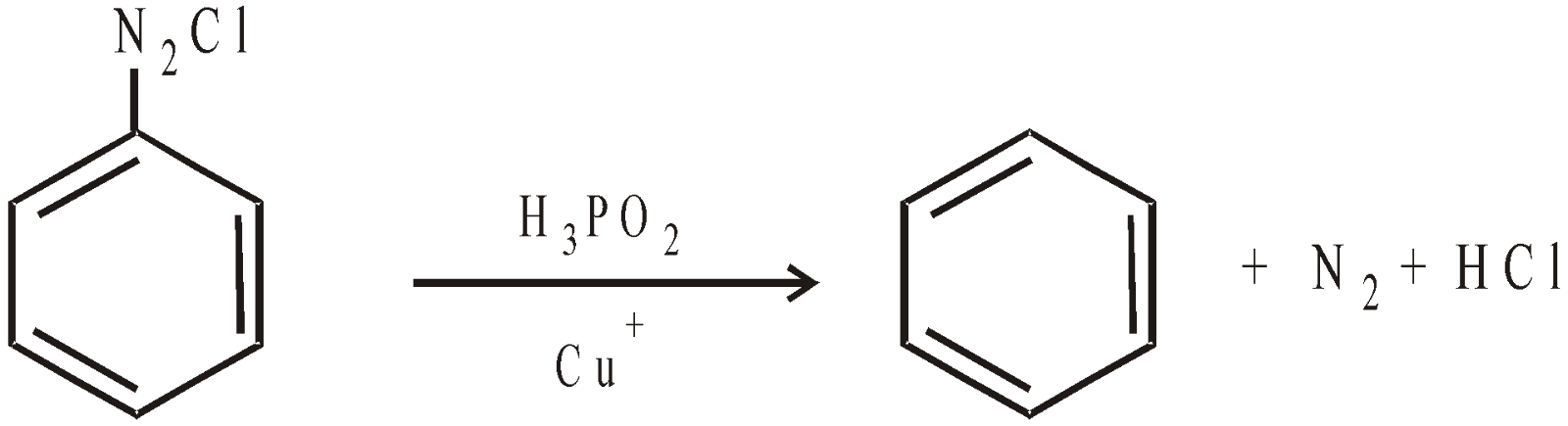
LARGE SCALE PREPARATION
-
From Petroleum : n-hexane fraction of petroleum

 + 4H2
+ 4H2
-
From light-oil fraction of Coal tar
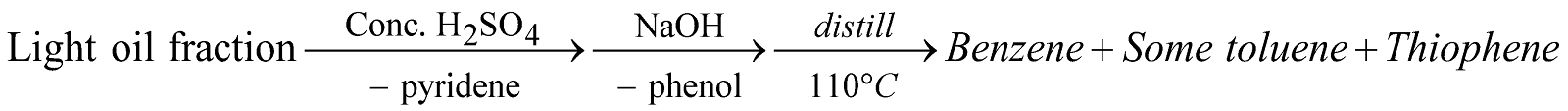
 Impurity of thiophene is removed by heating with hydrogen under pressure at 400°C in presence of catalyst.
Impurity of thiophene is removed by heating with hydrogen under pressure at 400°C in presence of catalyst.

PROPERTIES
Colourless Liquid : bpt 80.1°C. Insoluble in H2O.
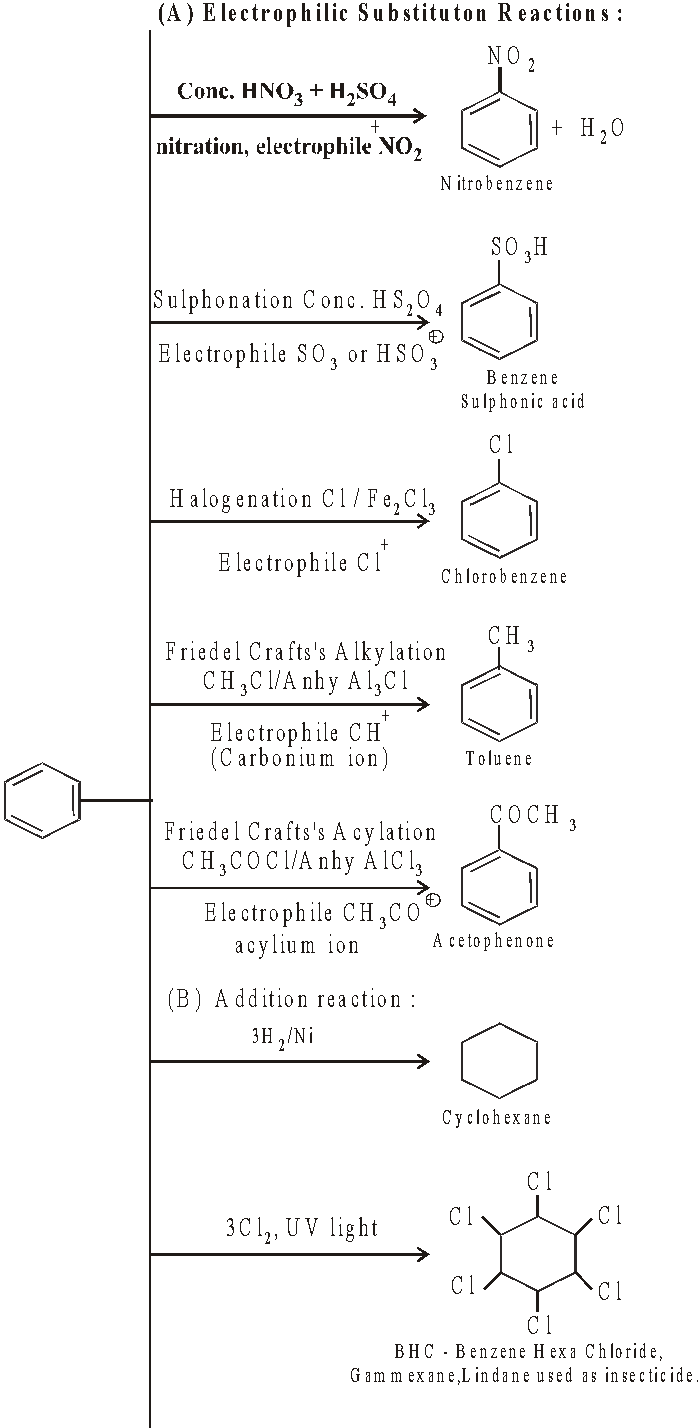
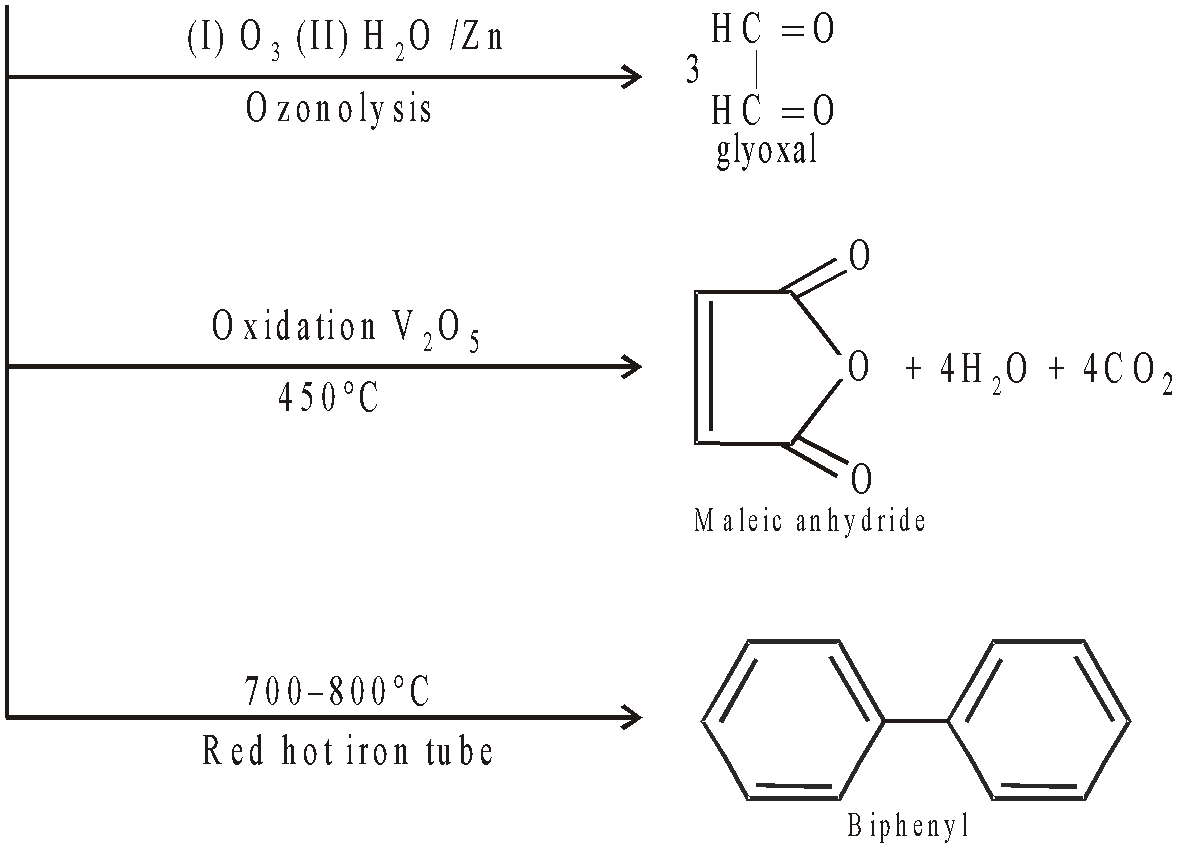 STRUCTURE OF BENZENE :
STRUCTURE OF BENZENE :
-
Open chain structures proposed.
-

-
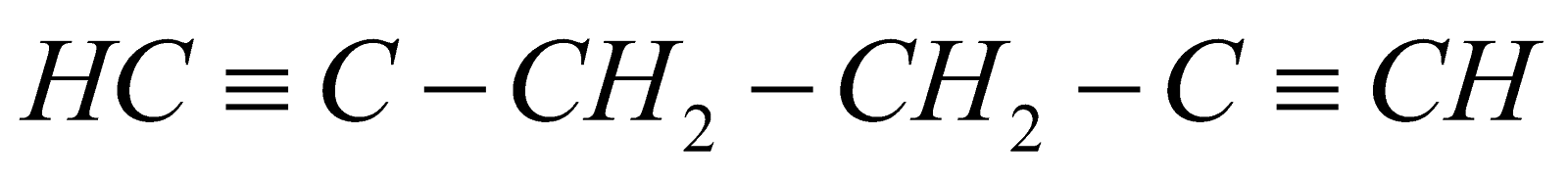
-
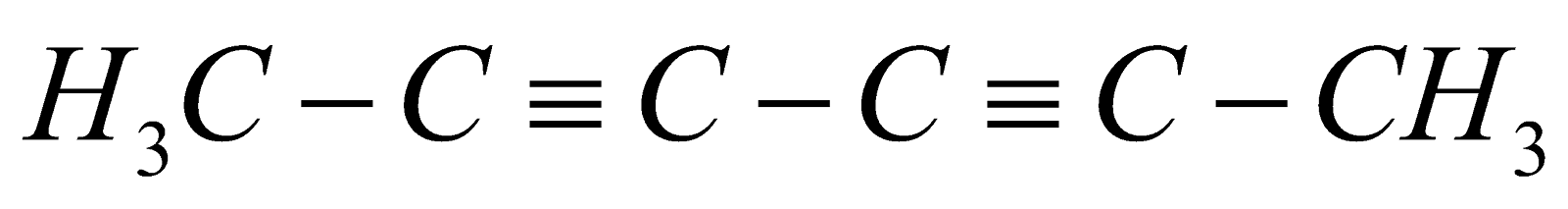
-
Ring structure by Kekule
 Objections against Kekule structure :
Objections against Kekule structure :
-
Two ortho di substituted derivatives
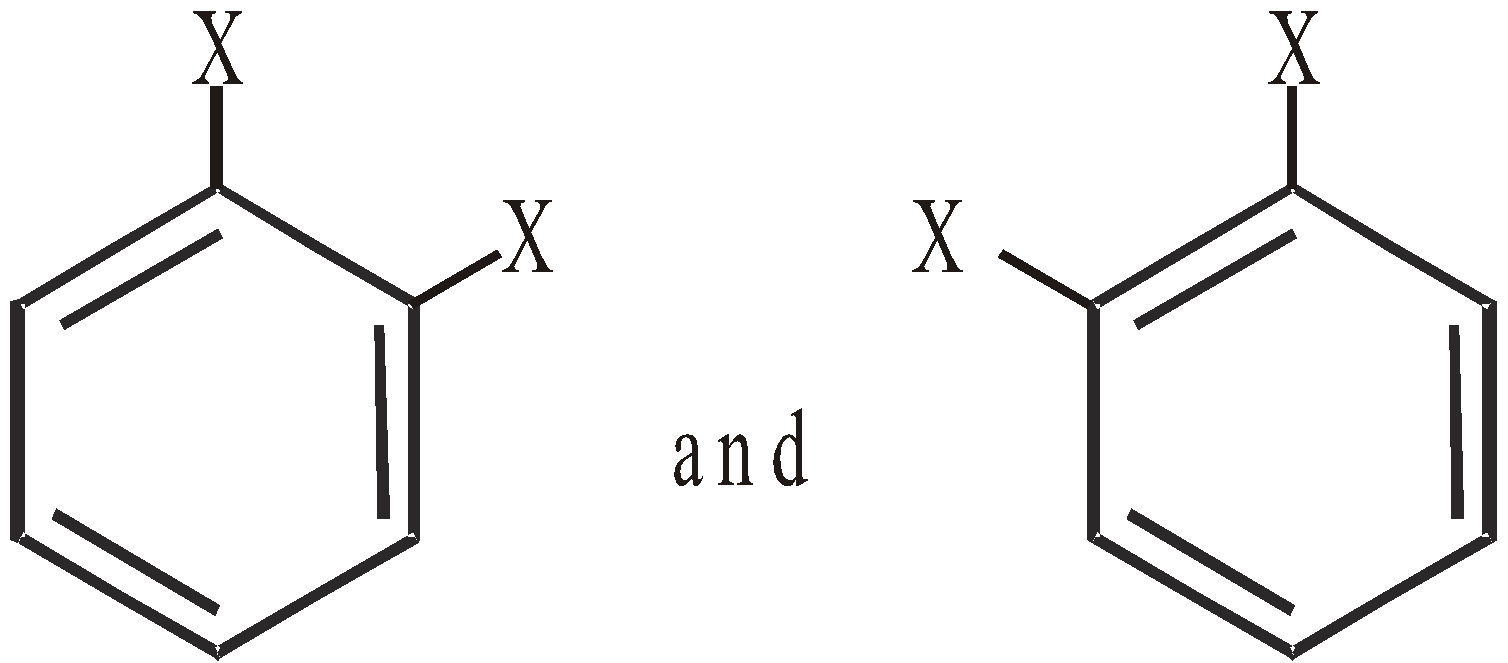
-
About Stability
Explanation against above objections :
Presence of two structures undergoing quick interconversion.

-
Levine and Cole : Levine and Cole confirmed the presence of above structures by ozonolysis of o-Xylene and obtaining the products.
-
Dimethyl glyoxal
-
Methyl glyoxal and
-
Glyoxal
-
Ladenburg's prism structure :
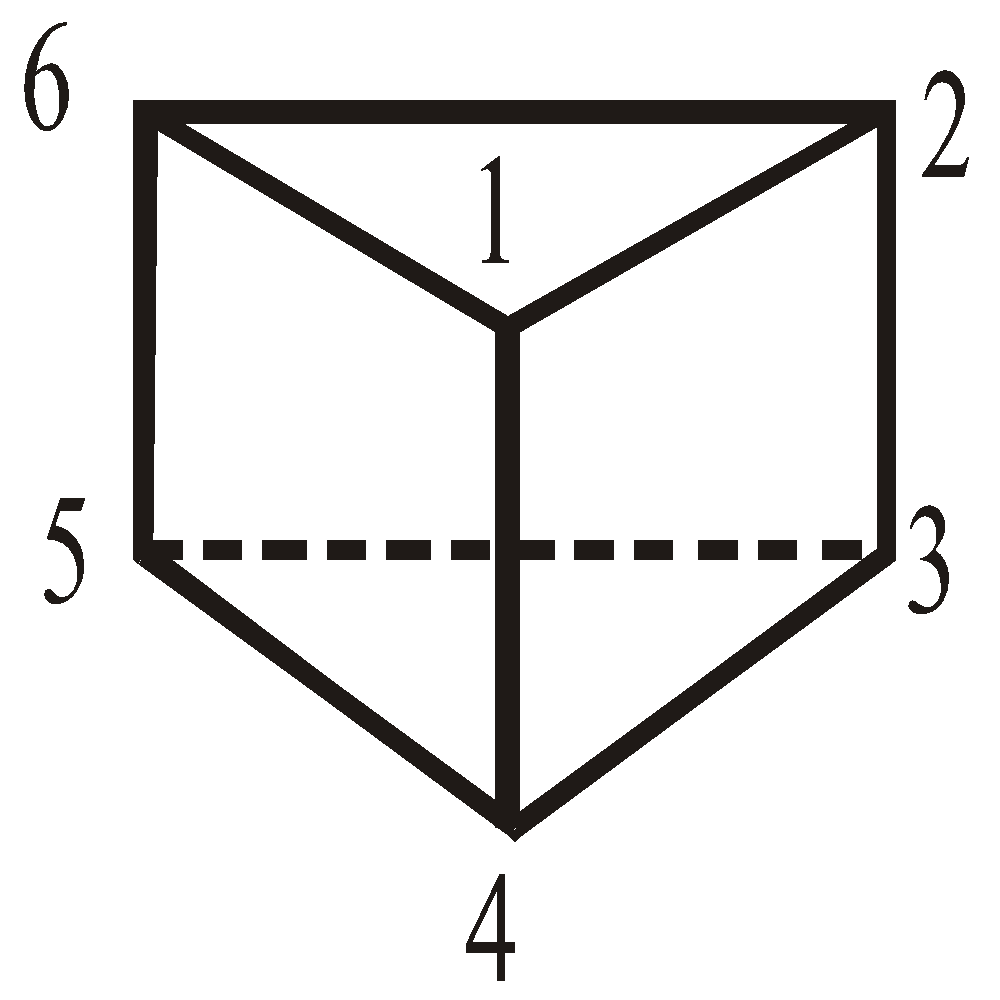 X-ray crystallographic methods showed benzene a planar compound whereas above structure is non planar.
X-ray crystallographic methods showed benzene a planar compound whereas above structure is non planar.
-
Claus and Dewar's structures :

-
Baeyer and Armstrong's centric structure :
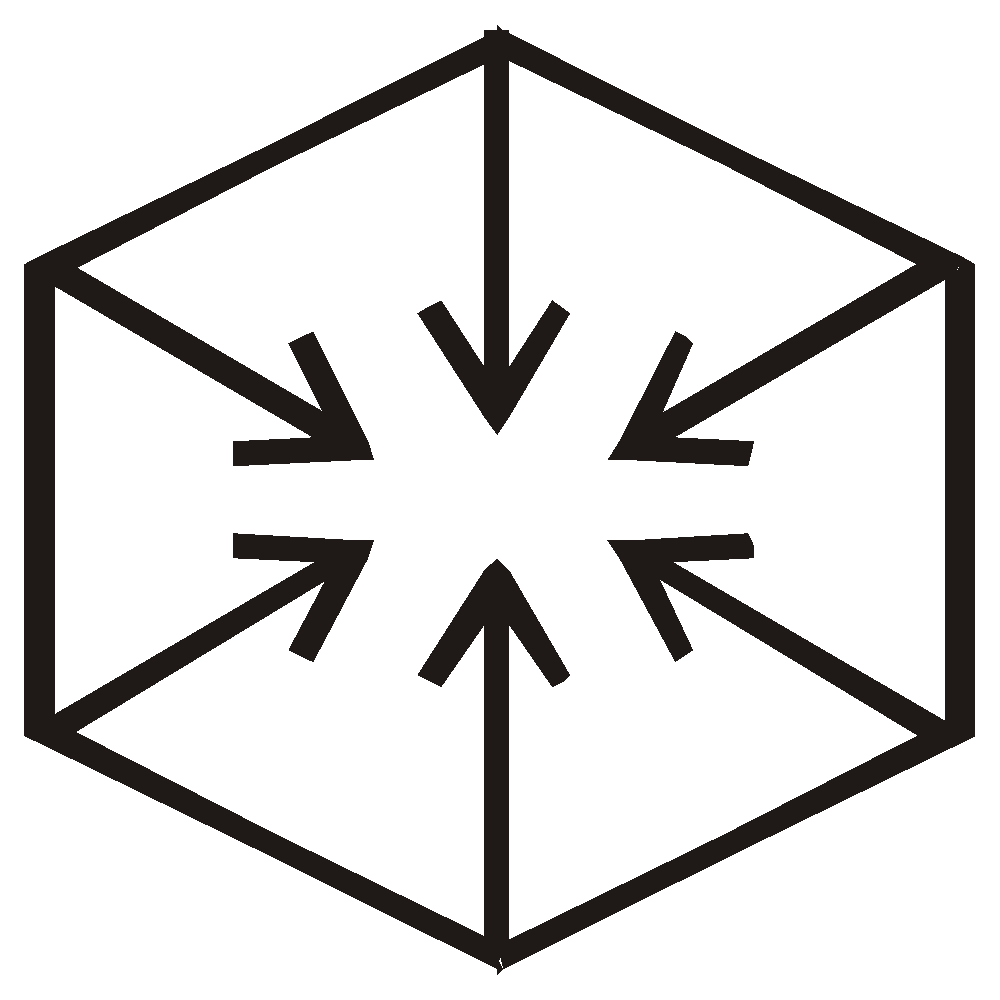
-
Robinson's sixtet structure
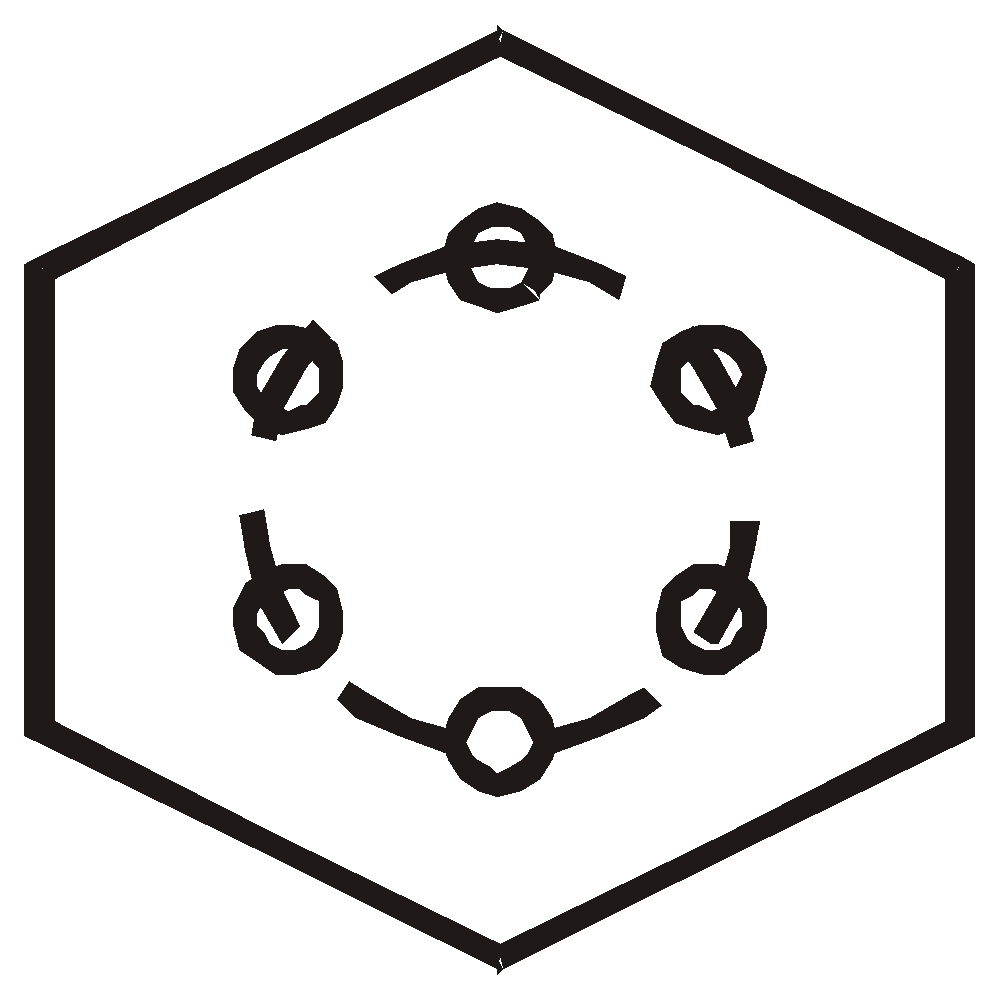 :
:
-
Thiele's structure of partial valency
 :
:
-
Benzene and resonance : Benzene is a resonance hybrid of the following structures.
 All C–C bonds in the benzene are of the same length 1.39Å.
All C–C bonds in the benzene are of the same length 1.39Å.
-
Benzene and aromaticity : Benzene is an aromatic compound since it obeys Huckel rule of (4n + 2)p electrons. The value of n must be an integer.
4n + 2 = 6p electrons of benzene
-
Molecular orbital structure of benzene : Each C-atom in benzene is sp2 hybridised and forms 3s bonds. The pz atomic orbitals left on each C-atom form a delocalised p-molecular orbital which stabilises the structure and renders all C-C bonds equal in length.


PETROLEUM
PETROLEUM
(petra = rock, oleum = oil). A thick dark coloured complex liquid, mixture of organic compounds obtained from below the surface of the earth is petroleum. The chief components of petroleum are hydrocarbons, aliphatic, alicyclic (naphthalenes) or aromatic in varying proportions and 1 to 6 percent of Sulphur, Nitrogen and Oxygen compounds.
NATURAL GAS
Found along with petroleum and roughly contains 60 to 80 percent methane 5 to 9 percent ethane, 3 to 18 percent propane, 2 to 14 percent higher hydrocarbons. It is used as fuel.
Partial combustion of natural gas yields Carbon blocks (reinforcing agents for rubber).
THEORIES OF ORIGIN
MENDELEEV'S CARBIDE THEORY

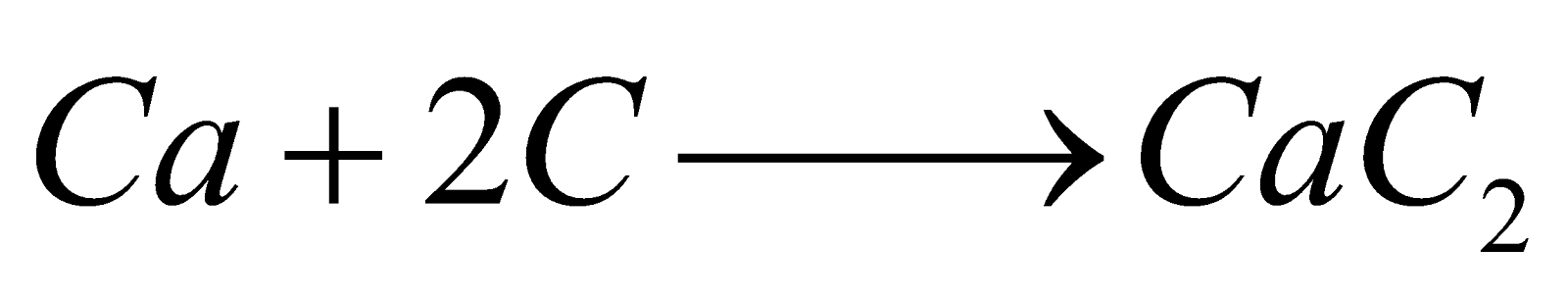



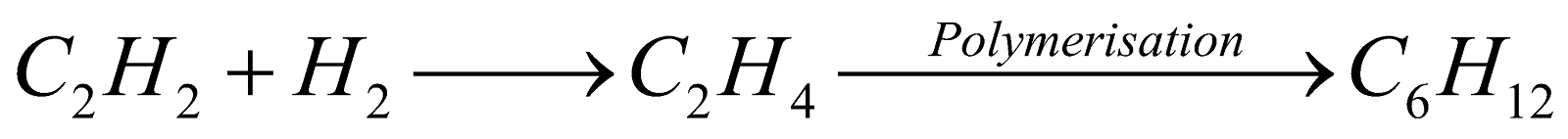 The theory was supported by Moissan, Sabatier and Senderens. It fails to explain the presence of optically active compounds, Compounds of N and S, chlorophyll and haemin derivatives.
The theory was supported by Moissan, Sabatier and Senderens. It fails to explain the presence of optically active compounds, Compounds of N and S, chlorophyll and haemin derivatives.
ENGLER'S THEORY
Petroleum is a product of slow decomposition of dead marine animals under high temperature and pressure. It explains the presence of brine, fossils, compounds of N and S.
It explains the presence of all the above mentioned compounds including chlorophyll. Hence petroleum is of animal as well as vegetable origin.
MODERN THEORY
Petroleum is produced by partial decomposition of marine animals and sea weeds etc.
Mining and Refining : Recovery from oil wells and separation of individual components.
FLASH POINT
The minimum temperature at which an oil gives off sufficient vapours to form an explosive mixture with air is called Flash Point.
KNOCKING
A sharp metallic sound emitted by internal combustion engine owing to immature ignition of the air gasoline mixture. Knocking is maximum in presence of straight chain hydrocarbons and minimum in presence of aromatic and branched chain hydrocarbons.
Anti Knock Compounds : 59% Tetraethyl lead, 13% Ethylene bromide, 24% Ethylene chloride, 4% Kerosene and dye is anti knock mixture.
In presence of aromatic compounds tetramethyl lead is more effective.
OCTANE NUMBER
The percentage of isooctane (2, 2, 4-trimethyl pentane) in a mixture of iso octane and n-heptane having the same knocking properties as the fuel under consideration.
The higher the octane number, the better is the fuel.
Octane number of compounds : Aromatic Compounds > Cyclo alkanes > Olefins > Branched chain alkanes > Straight Chain alkanes.
CETANE NUMBER
It is the percentage of cetane (n-hexadecane) in a cetane and a-methylnaphthalene mixture that has the same ignition qualities as the fuel.
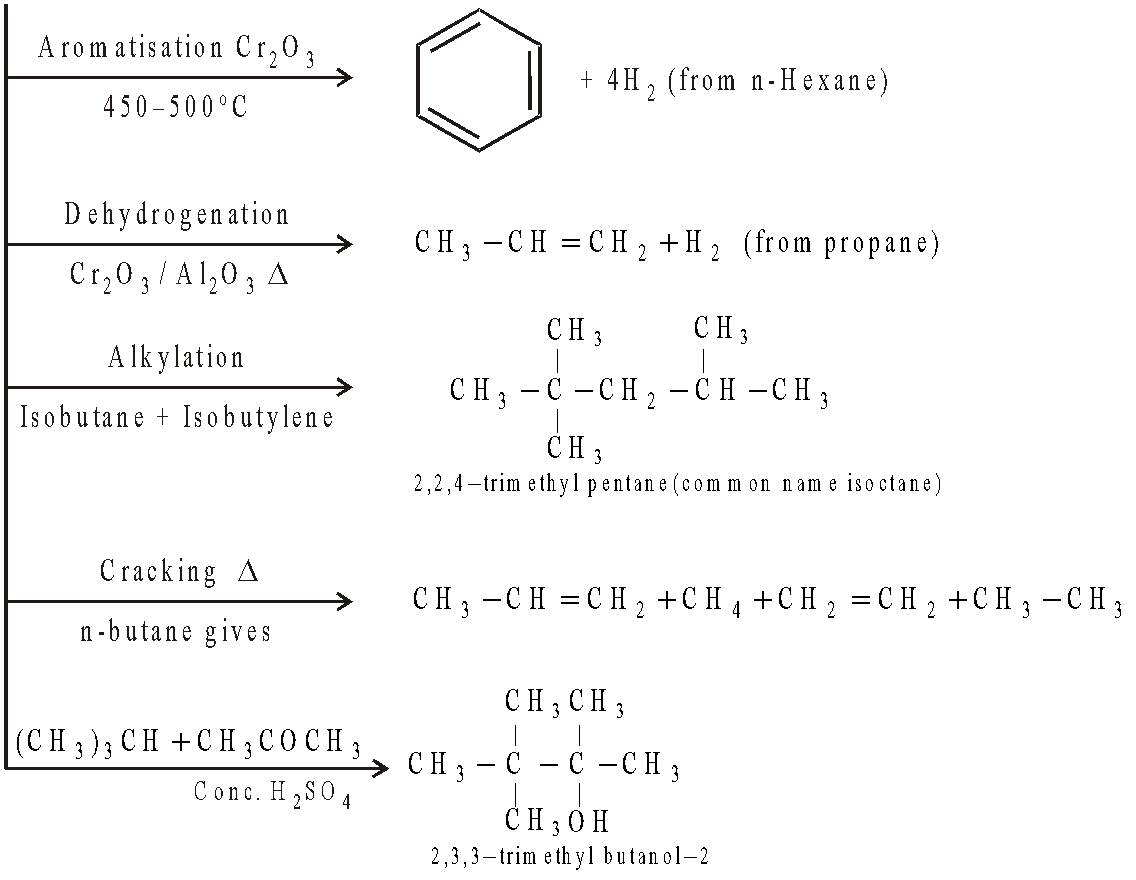
CRACKING
The conversion of less valuable higher fraction to the more valuable lower fraction by the application of heat is known as cracking.
-
Liquid Phase Cracking :
 Catalyst : Silica, titanium dioxide, zinc oxide, ferric oxide, alumina etc. High pressure keeps the oil in liquid state. Octane number of product : 65-70.
Catalyst : Silica, titanium dioxide, zinc oxide, ferric oxide, alumina etc. High pressure keeps the oil in liquid state. Octane number of product : 65-70.
-
Vapour phase Cracking :
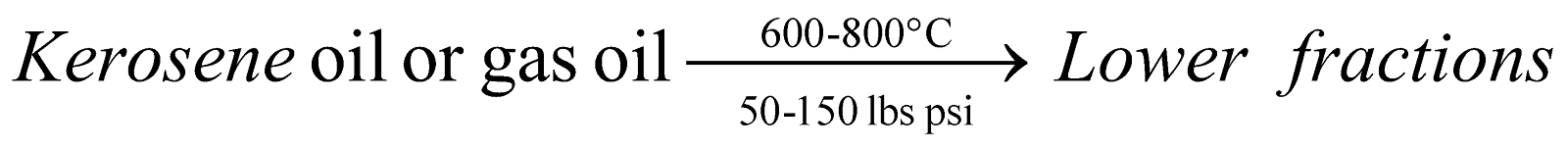
-
Cracking in presence of hydrogen :

Reactions taking place during cracking :
-
Carbonisation :

-
Dehydrogenation :

-
Polymerisation :

-
Alkylation :
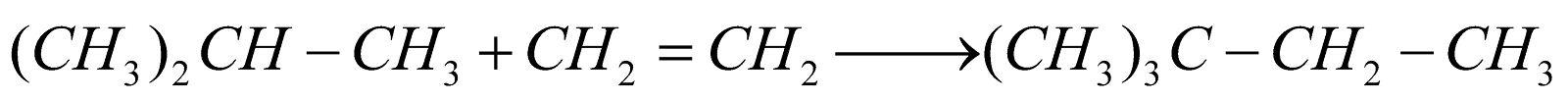
-
Aromatisation :
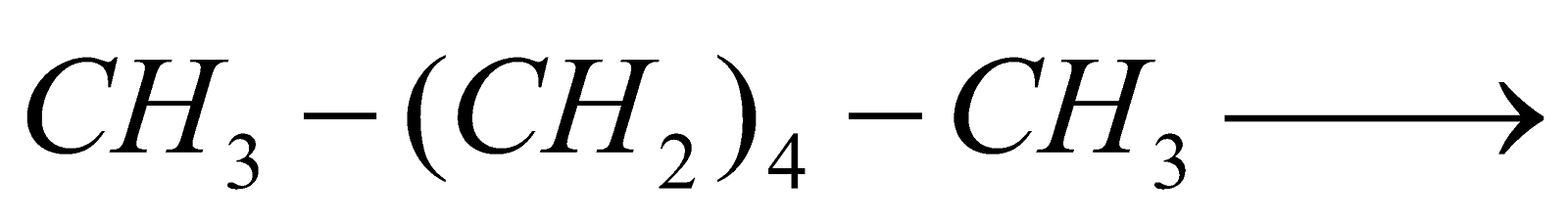
 + 4H2
+ 4H2
-
Chain Fission :
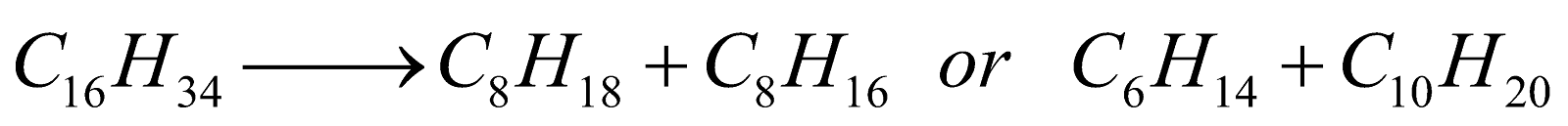
SYNTHETIC PETROL
BERGINS PROCESS
 Heavy oil is reused.
Heavy oil is reused.
FISCHER-TROPSCH PROCESS
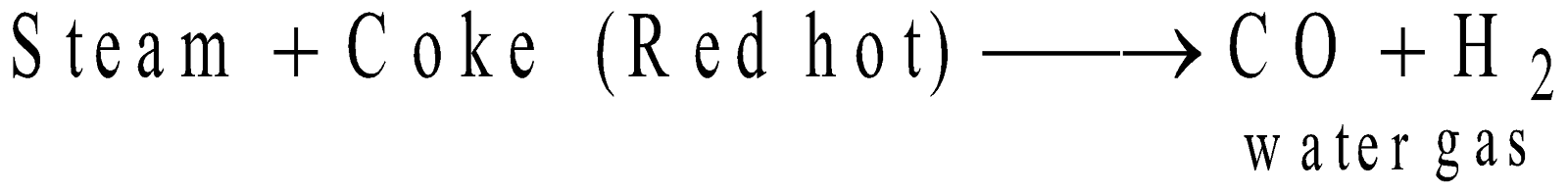

 Catalyst - Cobalt (100 parts), thoria (5 parts), magnesia (8 parts), Kieselguhr (200 parts).
Catalyst - Cobalt (100 parts), thoria (5 parts), magnesia (8 parts), Kieselguhr (200 parts).
REFORMING
It is increasing of antiknock properties by special type of cracking which includes Alkylation, Isomerisation, Aromatisation, Cyclisation, Dehydrogenation, Fractionation etc.
No lead petrol : It does not contain lead and obtained by reforming.
Petro chemicals : Chemicals derived from petroleum sources.
- Dehydrohalogenation and dehydration follows Saytzeff's rule (Hydrogen is removed from C-atom containing lesser number of H-atoms or more substituted alkene is formed)
- More substituted alkenes are more stable.
- Ease of dehydrohalogenation 3° > 2° > 1°.
- Ease of dehydrohalogenation : Iodides > Bromides > Chlorides
Formula
|
Common Name
|
Derived name
|
IUPAC name
|
CH ☰ CH
|
Acetylene
|
Acetylene
|
Ethyne
|
CH3C ☰ CH
|
Allylene
|
Methyl acetylene
|
Propyne
|
CH3C ☰ C– CH3
|
Cretonylene
|
Dimethyl acetylene
|
But-2-yne
|
- Dimethyl glyoxal
- Methyl glyoxal and
- Glyoxal
