Bringing the World's Best Biology to You
Free Chemistry NEET Video Lectures for Structure of Atom
Exams: NEET / IIT-JEE
Audience:
- General Public
- Educators of H. School / Intro Undergrad
- Educators of Adv. Undergrad / Grad
- Student
- Researcher
- Educators
Teachers: Masters / Phd. Holders
VIDEO LECTURES FOR STRUCTURE OF ATOM (CLASS 11)
NEET CHEMISTRY VIDEOS
07 Free Chemistry NEET Video Lectures available for Structure of Atom (Neet Chemistry)
Power up your NEET Exam prep with 07 Chapter-Wise Chemistry Video Lectures for Structure of Atom at Onlineneetcoaching.in. Crafted by Brilliant Tutorial experts with 25 years of NEET insights.
Related Videos
Demo Video Lecture 01
Structure Of Atom | Video #1 | NEETClass 11th Chemistry Video Lecture
Demo Video Lecture 02

Structure Of Atom | Video #2 | NEETClass 11th Chemistry Video Lecture
Video Lecture 03

Structure Of Atom | Video #3 | NEETClass 11th Chemistry Video Lecture

Video Lecture 04
Structure Of Atom | Video #4 | NEETClass 11th Chemistry Video Lecture

Video Lecture 05

Structure Of Atom | Video #5 | NEETClass 11th Chemistry Video Lecture

Video Lecture 06

Structure Of Atom | Video #6 | NEETClass 11th Chemistry Video Lecture

Video Lecture 07
Structure Of Atom | Video #7 | NEETClass 11th Chemistry Video Lecture

Why Excel in Structure of Atom for NEET Exam
Overview
NEET Class 11 Chemistry NCERT Chapter 2 Structure of Atom
In this video, we will cover:
Chapter 2 Structure of Atom Class 11 NEET Chemistry deals with:
- As we have already studied about Daltons Atomic Theory of Matter, and later it was concluded that Atom is made up of 3 sub -atomic particles.
- The particles are Electron, Proton and Neutron.
- In this chapter ,we will study about different attempts made to explain atomic models.
- The phenomenon that leads to development of dual nature of Light and Matter.
- Also, we will deal with the methods to be followed while writing electronic configurations, and much more.
- Let us see that first how these fundamental particles were discovered, and how they are arranged in structure of Atom.
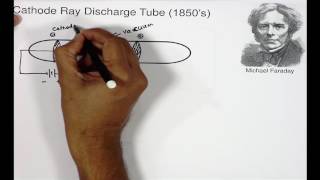
DISCOVERY OF ELECTRON:
- The Electron was discovered by J.J Thomson by conducting a Cathode ray tube experiment.
- For the experiment he used Crooke’s tube, which was 60cm long glass tube and had a small tube attached.
- To this small tube vacuum pump was attached, it also had two metal plates which were connected to battery by wires.
- The tube contained gas at atmospheric pressure. when current at high voltage (10,000volts) was passed following observations were made:
- When current was passed through a gas at 1 atmospheric pressure and at a very high voltage, nothing happened. That is no visible effect was seen inside the tube.
- Then further the pressure of gas was reduced by pumping the air out, with the help of vacuum pump. The pressure was reduced to 10-2atm, then on passing current it was seen that whole tube started glowing green.
- Then further the pressure was reduced to 10-4It was seen the whole glow vanished, but it was seen that at the end of the tube (anode side) there was a faint green glow observed.
- To confirm the faint glow anode was made perforated, and a zinc sulphide screen (fluorescent material) was placed behind it.
- When current was passed under same conditions it also started glowing green.
- This confirmed that under those conditions some rays were emitted through cathode, and were travelling towards anode.
- Those rays were called as cathode rays and found to consist of negatively charged particles called electron.
Properties of cathode rays
- They are found to travel in straight line. This property was concluded by performing an activity where the object was placed in their path. When they strike it ,they casted the shadow of the object as shown below:
- Cathode rays are formed of material particles. This property was concluded by performing an activity where in their path a paddle was mounted on an axle.When they strike it ,they rotated the paddle .
- They are negatively charged.This property was concluded by performing an activity where electric and magnetic field was applied, they deflected towards positive plate and in presence of magnetic field they deflected towards north pole.
- ❯ When they strike some metal surface, they heated it. So, it was concluded they can also produce heating effect.
- ❯ They cause ionization of gas through which they passed.
- ❯ They produce x-rays when strike against the hard metal like tungsten etc.
- ❯ They can make florescent material glow, when strikes some fluorescent material.
- ❯ They affect the photographic plate.
- ❯ They have penetrating effect.
From the above properties and experiment it was concluded that cathode rays are made up of negatively charged particles called electron.
You can practice DPPs, Download PDF of Solutions of DPPs, Revise notes for NEET Class 11 Chemistry Chapter 2 Structure of Atom.
Feel free to take a test on Class 11 Structure of Atom Chapter 2 NEET Chemistry, and test your understanding.
If you liked our content on Structure of Atom NEET Chemistry Chapter 2 Class 11, SHARE with your friends!
All About: Structure of Atom NEET Chemistry NEET Chapter 2
NEET Chemistry seems to be a quite difficult subject for a lot of students. But, if you get a very good conceptual understanding of the subject, it can be very interesting for you. We, at Brilliant Tutorials Delhi, will give our best to make NEET Chemistry Structure of Atom NEET Chapter 2 super-duper easy for you. Learn about Structure of Atom NEET Chemistry in detail with experts at Onlineneetcoaching.in is what we promise. Learning is a lifelong process. As a student, your focus should always be on learning new facts, concepts and tricks so that you can apply them in real-life scenarios. If your conceptual understanding is clear, you will anyway perform well in your exams. So, please do not just study for the fear of exams or just to score marks, instead learn for the love of learning. We have seen many students mugging up concepts without understanding, which, in turn, makes the subject more difficult and boring for them in the future. Always remember, if your basics are strong, you will be able to understand even the most complex concepts.
We aim at making learning fun as well as engaging for you with our complete end-end learning content with Structure of Atom NEET Chemistry Best videos, NEET pdf download, Notes, complete syllabus of the textbook, tests, and Practice Questions. Onlineneetcoaching.in (formerly called ExamFear Education) is a Free Education platform with more than 10,000 videos on Physics, Chemistry, Maths, Biology, English, Science experiments, tips & tricks, and motivational videos for Classes 6 to 12, NEET, JEE. Learn for Free!
We, at Brilliant Tutorials Delhi, will provide you with the best quality videos on NEET Chemistry Structure of Atom with special focus on concepts and a lot of examples, also you can download pdf. Not just that, a quick easy-to-refer and understand Structure of Atom NEET Chemistry NEET Chapter 2 Notes, based on the revised NCERT textbooks. You can completely rely on the study materials created by our experts and can definitely score very well in your exams. You also have the option to download pdf as well as complete all topics of the textbook. All of these mentioned above will be accompanied with:
- NEET Chemistry Structure of Atom NEET Chapter 2 DPPs: A set of 20 questions from each chapter for you to attempt and check your understanding. Solutions are also available and can be downloaded in pdf format. Download Structure of Atom NEET Chemistry NEET Chapter 2 DPPs solutions PDF for free. Practice these questions once you have watched the videos of the chapter; try to answer on your own. Once done, refer to the solutions to check if you were right or not. This would improve your problem-solving skills as well as help you revise topics you are less confident with. In fact, our research says that the majority of the students feel that their concepts are clear while attending a class or watching a lecture video. But it is during solving a problem or numerical that they truly get to know if they have understood the concept or not. Therefore, we strongly recommend you to solve the DPPs as soon as you have watched all the videos of that chapter.
- NEET Structure of Atom Online Tests: For every chapter, you can take a test of 10 Multiple choice questions. Correct answers along with explanations will be provided after you submit the test. A set of new 10 questions will appear when you try to attempt the test for the same chapter again. This will help you to revise your concepts on Structure of Atom NEET Chemistry. Self-evaluation is one of the best ways for you to understand how much clarity you have got on the concepts of that topic. Based on where you are getting stuck, you can give extra focus on those topics. Taking tests frequently always helps you improve your speed and accuracy.
- Structure of Atom NEET NEET solutions: All questions from NEET textbook are solved in detail, along with video solutions for each question. Download Structure of Atom NEET NCERT solutions PDF for free. Students often get stuck while solving the questions in the exercises of NEET textbook. You can watch the NEET solutions in the form of videos with our experts, and can also refer to pdf format on our website as well as app. If you are a student of CBSE or preparing for exams for NEET, JEE, Civil services, NDA etc. NEET textbooks are very important for you. Please note that we have uploaded videos as per the Revised NEET textbooks (2023-24). Therefore, you can completely rely on the content on our website/app.
- NEET Chemistry Sample papers: You must attempt a couple of our specially curated sample papers before appearing for your exam. This will boost your confidence and also help you practice. Once you have completed the syllabus, it is very important for you to solve Sample papers. In an exam-like environment, solve the sample paper. Once done, check the answers and understand which are your strong areas and which are the weaker ones. The sample papers are created based on the complete syllabus of a particular subject.
- NEET Chemistry Previous Years’ Questions: Questions that appeared in the last 5-7 years along with detailed solutions for better understanding for the entire NEET Chemistry syllabus. This is a very critical step towards your preparation for any examination. This gives you a fair idea of what kind of questions are asked in that particular exam. It also helps you understand the important topics from where the majority of questions are framed. We will provide you with Previous years’ questions of Class 10 and Class 12 NEET exams, as well as NEET, JEE, both in video format as well as PDF format.
- Last but not least, We, at Brilliant Tutorials Delhi, are on a mission to Neet Education.
If you like our content, Spread the word!
Tips for Students- Never miss a video on Structure of Atom NEET Chemistry
- Make your notes while watching Structure of Atom Class 11 NEET Concept Add on Electron, Proton & Neutrons, Atomic Models| Chemistry
- Make a note of all your doubts as you watch Structure of Atom Class 11 NEET Concept Add on Electron, Proton & Neutrons, Atomic Models| Chemistry, and ask them in the “Ask Question” section of Onlineneetcoaching.in
- How should I prepare for my NEET Chemistry exam?
- Watch all our Videos, Notes, NEET Solutions, DPPs for NEET Chemistry
- Download PDF of Solutions, complete syllabus of textbooks is covered in the videos
- Attend LIVE classes to ask your doubts on NEET Chemistry
- Can I crack NEET/JEE/Board exam using Brilliant Tutorials Delhi?
Of course, YES! If you are determined to crack NEET, you can at ease do that with Onlineneetcoaching.in
- Watch NEET Videos on Physics, Chemistry, Biology
- Ask your doubts in NEET/JEE/Board exam LIVE classes
- Refer Notes, DPPs, Online Tests, Practice questions, sample papers, DPPs for NEET
- Can I get a good score in NEET Chemistry board exam if I study only from Brilliant Tutorials Delhi?
Yes, you can. Refer Structure of Atom NEET Chemistry NEET Chapter 2 videos, notes, tests, Daily practice problems, questions, sample papers. Similarly, cover all chapters of the complete syllabus of NEET for NEET Chemistry
- How to make use of Brilliant Tutorials Delhi the best possible way to understand Structure of Atom NEET Chemistry NEET Chapter 2?
- Step 1. Watch all videos, without skipping any video for Structure of Atom NEET Chemistry
- Step 2. Ask your doubts in Ask questions section of Onlineneetcoaching.in
- Step 3. Try DPPs, Revise notes, Download PDF for DPPs solutions, refer NEET solutions for Structure of Atom NEET Chemistry
- Step 4. Take online test, sample paper for Structure of Atom NEET Chemistry
- Can I ask my doubts on Brilliant Tutorials Delhi?
Yes. You can ask your doubts in the Ask Question section of Onlineneetcoaching.in. Alternatively, you can also ask questions on our Android Brilliant Tutorials Delhi app. We will try to answer your questions within 24 hours.
- Are all content on Brilliant Tutorials Delhi free? or some courses are paid as well?
Brilliant Tutorials Delhi is a Free learning platform. Brilliant Tutorials Delhi provides the best videos, notes, pdf, tests, practice questions, DPPs absolutely free of cost. Hope you have watched Free videos on Structure of Atom NEET Chemistry. Did you like them? Brilliant Tutorials Delhi
If you like the video on Structure of Atom Class 11 NEET Concept Add on Electron, Proton & Neutrons, Atomic Models| Chemistry ❯  For Premium Package
For Premium Package 
Structure of Atom FAQs
How many Video Lectures are there for Structure of Atom?▶
Onlineneetcoaching.in offers 20 free NEET Video Lectures for Structure of Atom, each with 10-20 questions—200-300 total. Crafted from 25 years of NEET trends, they cover all 2-3 questions this chapter brings to NEET Exam!
Are the NEET Video Lectures free?▶
Yes, all 20 Structure of Atom NEET Video Lectures are 100% free—no fees, no catch. Expert-designed with 25 years of NEET insights, they’re open to all aspirants at Onlineneetcoaching.in.
What topics do these Video Lectures cover?▶
Our 20 NEET Video Lectures span Structure of Atom’s syllabus: atomic models (Bohr, Rutherford), quantum numbers, spectral series (e.g., Balmer n₂ = 3 to n₁ = 2), de Broglie wavelength, photoelectric effect—everything NEET Exam tests.
Do the Video Lectures come with solutions?▶
Absolutely! Each of our 20 NEET Video Lectures includes instant, detailed solutions—e.g., calculate Bohr energy (Eₙ = -13.6 Z²/n²) for He⁺ or spectral wavelength. Learn and fix mistakes fast.
Can I practice under NEET exam conditions?▶
Yes, our 20 timed NEET Video Lectures mimic the real exam—45 Chemistry questions, ~1 minute each. Perfect for mastering Structure of Atom’s 2-3 questions under pressure.
Are these Video Lectures updated for NEET Exam?▶
Definitely! Our 20 NEET Video Lectures are refreshed weekly for NEET Exam, focusing on Bohr’s model, quantum numbers, and more—no syllabus cuts noted, just pure numerical and conceptual prep.
What’s Structure of Atom’s weightage in NEET?▶
Structure of Atom carries 2-3 questions (8-12 marks) in NEET’s 180-mark Chemistry section. Our 20 mock tests ensure you nail every point with targeted practice.
How do 25-year trends shape these tests?▶
Our experts analyze 25 years of NEET questions—like 2022’s quantum number set or 2023’s photoelectric V₀—to craft our 20 Video Lectures, matching the exam’s style and difficulty.
How can I ace Structure of Atom with these tests?▶
Practice all 20 NEET Video Lectures (200-300 questions)—focus on Bohr energy, spectral lines, and quantum rules. Review solutions (e.g., λ = h/mv errors), and build speed with our timed format.
Why pick Onlineneetcoaching.in for Structure of Atom?▶
Onlineneetcoaching.in delivers 20 free NEET Video Lectures for Structure of Atom, each with 10-20 questions, rooted in 25 years of NEET data. Free, updated, and expert-crafted—they’re your edge for NEET Exam 2-3 questions.